Figure 1.
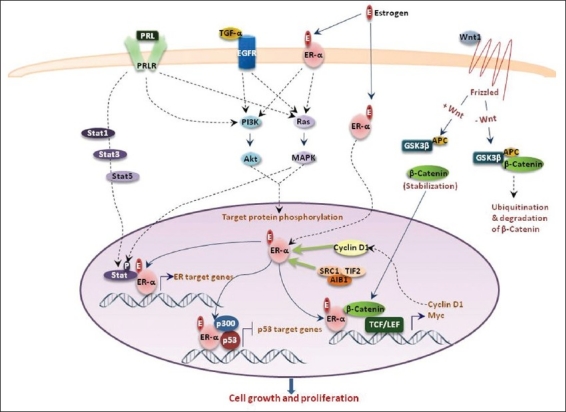
Estrogen functions through multiple pathways. Binding of Estrogen (E) to the Estrogen Receptor-α (ER-α) leads to translocation of the ligand receptor complex to the nucleus, where it affects transcription of an independent set of genes.[95] In addition, ER also exerts its effect on growth and proliferation of cells by binding to and affecting transactivation activity of various growth-related transcription factors (TF). Binding of the Wnt ligand to frizzled receptor leads to stabilization of β-catenin and its subsequent translocation to the nucleus, where it binds to TCF / LEF TF and drives its target genes. β-catenin activity in the nucleus is modulated by ER, leading to enhanced transactivation of the Wnt target genes.[96] Binding of estrogen to the membrane-bound ER activates downstream signaling pathways that include PI3K and Ras-MAPK pathways.[95] Transforming growth factor alpha (TGF-α) binds to its receptor, the epidermal growth factor receptor (EGFR), leading to its activation and a subsequent transcription of proliferative genes, through activation of PI3K and Ras-MAPK signaling.[21] Prolactin (PRL) binds to its trans-membrane cell-surface receptor, the prolactin receptor (PRLR), and triggers a tyrosine kinase-mediated signaling cascade, which leads to the activation of Stat1, Stat3, and Stat5, leading to their translocation to nucleus, where they bind to their target gene promoters.[97] ER binds to Stat target genes and enhances the transcriptional activity of Stats. Additionally, cyclin D1 binds directly to the hormone-binding domain of the estrogen receptor, resulting in an increased binding of the receptor to estrogen response element sequences, and upregulates ER-mediated transcription.[98–99] Similarly, AIB1 in complex with Src1 and TIF2, potentiates transcription of ER-regulated genes.[100] Finally, ER has been shown to bind to p53 on the p53 target gene promoters in breast cancer cells, leading to repression of the p53 transactivation function.[101–102]
