Abstract
Context:
Influence of timing of repair on outcome following laparoscopic reconstruction of lower ureteric strictures
Aims:
To assess the influence of timing of repair on outcome following laparoscopic reconstruction of lower ureteric strictures in our adult patient population.
Settings and Design:
Single surgeon operative experience in two institutes. Retrospective analysis.
Materials and Methods:
All patients were worked up in detail. All patients underwent cystoscopy and retrograde pyelography prior to laparoscopic approach. Patients were categorised into two groups: early repair (within seven days of inciting event) and delayed repair (after two weeks). Operative parameters and postoperative events were recorded. Postprocedure all patients were evaluated three monthly. Follow-up imaging was ordered at six months postoperatively. Improvement in renal function, resolution of hydronephrosis and unhindered drainage of contrast through the reconstructed unit on follow-up imaging was interpreted as a satisfactory outcome.
Statistical Analysis Used:
Mean, standard deviation, equal variance t test, Mann Whitney Z test, Aspin-Welch unequal variance t test.
Results:
Thirty-six patients (37 units, 36 unilateral and 1 simultaneous bilateral) underwent laparoscopic ureteral reconstruction of lower ureteric stricture following iatrogenic injury - 21 early repair (Group I) and 15 delayed repair (Group II). All patients were hemodynamically stable at presentation. Early repair was more technically demanding with increased operation duration. There was no difference in blood loss, operative complications, postoperative parameters, or longterm outcome.
Conclusions:
In hemodynamically stable patients, laparoscopic repair of iatrogenically induced lower ureteric strictures can be conveniently undertaken without undue delay from the inciting event. Compared to delayed repairs, the procedure is technically more demanding but morbidity incurred and outcome is at par.
Keywords: Boari flap, iatrogenic, laparoscopy, ureteric stricture, ureteroneocystostomy
INTRODUCTION
Reconstruction of lower ureteric stricture is challenging due to precarious vascular supply of the lower ureter and the propensity to restenosis unless diligently handled.[1] In iatrogenic pathologies, immediate repair may be further complicated by the unfamiliar anatomy secondary to the inciting surgical event. The ureter may also be entrapped in dense fibrosis secondary to multiple prior restorative interventions or the sufferer may present with renal compromise that may complicate procedural performance. Additionally long segment lower ureteral pathologies demand flap reconstructions for satisfactory reestablishment of ureterovesical continuity. Accordingly surgical approaches for re-establishment of ureteral continuity are technically demanding and definitive repairs are usually favored after a period of six weeks from the inciting event.[2] In a hemodynamically stable sufferer this adds to the morbidity and impairs his functionality. Also traditionally ureteral reconstructions have been undertaken through incisional approach. This exercise is cosmetically unappealing and the procedural morbidity is considerable. Following the success in ablative procedures, laparoscopic approach has been increasingly employed for reconstruction of urological pathologies and for reconstruction of lower ureteral strictures, albeit sparingly.[3] Although the projected outcome following laparoscopic reconstruction is promising,[4] citations till date have not focussed on the feasibility of conducting this approach primarily after the inciting event. This paper aims to compare the immediate and long term outcomes following early and delayed laparoscopic repair of lower ureteric strictures.
MATERIALS AND METHODS
A retrospective analysis was undertaken to identify all laparoscopic reconstructions performed for lower ureteric strictures secondary to iatrogenic etiology from June 2006 till August 2010. All reconstructions performed in adult population were included in our study cohort. All procedures were performed by a single surgeon (GPA) at two different centers. A detailed history was elicited in all. Blood parameters including renal profile were evaluated. All patients underwent initial ultrasound evaluation. A 64 slice spiral computed tomography urogram (CTU) or intravenous urogram (IVU) was the preferred imaging for pathology delineation. A laparoscopic approach was attempted in all. Polyethylene glycol (Peglec) bowel preparation was ordered evening prior to laparoscopic approach. All patients underwent cystoscopy and retrograde dye delineation immediately prior to definitive correction. Subsequently patients were positioned in steep Trendelenberg decubitus with shoulder support. Four ports were employed [Figure 1]. Ports were modified as per previous surgical incisions or location of stomas [Figure 2]. The ureter was identified near the level of ipsilateral sacroiliac joint and confluence of iliac vessels. The dissection was continued caudally. Ureter was handled meticulously with preservation of periureteral adventitia. Dissection in proximity to the ureter was performed using cold scissors. The ureter was disconnected at a healthy margin away from the pathological segment. Ureter was spatulated at the six o clock position on the posterior margin. The bladder was then inflated with 200 milliliters (ml) of normal saline (0.9% weight/volume) through the preplaced perurethral catheter. Bladder was mobilized widely using harmonic scalpel (Ethicon, Cincinnati, USA). Anterior mobilization was carried out by division of the urachus and entering into the space of Retzius. Lateral mobilization was performed by division of the peritoneal reflection. The distance between the mobilized bladder and the transected ureter was assessed and decision for ureteroneocystostomy, additional psoas hitch or Boari flap ureteroneocystostomy was made accordingly. The technique of ureteroneocystostomy in our practice is mentioned below. A full thickness buttonhole cystostomy was conducted using harmonic scalpel. Ureter was approximated to bladder employing single layer full thickness interrupted suturing (3/0 polyglactin suture). A six F (French scale) ureteral stent was inserted in a retrograde fashion after completion of the posterior layer of the anastomosis. Additional psoas hitch was employed if tension was anticipated at ureterovesical anastomosis. The posterolateral wall of the bladder was anchored to the ipsilateral psoas tendon with interrupted sutures of 2-0 polyglactin for this purpose. A drain was inserted at completion of all procedures. Postoperatively patients were allowed orally once comfortable. Drain and catheter pull outs were scheduled as indicated. Ureteral stents were removed six weeks postoperatively. Operative parameters recorded were operation duration, blood loss, operative complications. Postoperative parameters recorded were time to oral tolerance, pain scores immediate post procedure (visual analog scale, 1-10, 1-minimum and 10 maximum), time to drain removal, analgesic need, hospital stay and any complications suffered during postoperative period. All patients were evaluated clinically as well as biochemically three monthly till one year. Renal profile and ultrasound evaluations were carried out at each follow-up visit. Subsequent follow-ups were scheduled as deemed. Contrast imaging was scheduled at six months postprocedure. Long term outcome parameters were clinical course since stent removal, renal function, ultrasound appearance and appearance on contrast imaging at six months. Absence of symptoms during follow-up period, stabilisation or improvement in serum creatinine, and unobstructed drainage of urine through the reconstructed unit with reduction in pelvicaliceal dilatation were perceived as satisfactory result. Statistical analysis was carried out calculating mean and standard deviation of each parameters. Comparisons between early and delayed repairs were undertaken using equal variance t test, Mann Whitney Z test, Aspin-Welch unequal variance t test.
Figure 1.
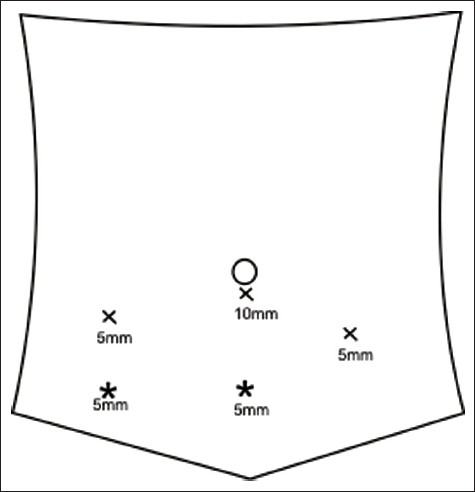
Port positions for laparoscopic ureteroneocystostomy and Boari flap. Asterix indicates possible positions for the fourth port (unilateral right side or bilateral pathologies)
Figure 2.

Modified port positions in patient with colostomy
RESULTS
Forty (41 units) laparoscopic reconstructions of lower ureteral pathologies (ureteroneocystostomy or Boari flap) have been successfully performed by the operator during this period. Thirty-six patients (37 units) reported an iatrogenic etiology and underwent laparoscopic ureteroneocystostomy with or without psoas hitch. Demographic and etiologic details are revealed in Table 1. One patient presented with bilateral vesicoureteric stenosis and vesicovaginal fistula following mesh suspension of vaginal vault. The mesh had migrated cranially and eroded into bladder trigone. Laparoscopic vesicovaginal fistula repair with simultaneous bilateral laparoscopic ureteroneocystostomies were undertaken. Another patient in our cohort attended with misplaced ureteral stent with lower ureteric stenosis following ureteroneocystostomy through open approach at a different centre. The misplaced stent was retrieved laparoscopically followed by laparoscopic ureteroneocystostomy with psoashitch. Mean time interval between inciting event and definitive laparoscopy was 14.24 days (range three days to two months). Twenty-one patients underwent early repair (within one week, Group I) and fifteen cases underwent delayed repair (after three weeks, Group II). All patients were hemodynamically stable with normal renal profile at presentation. All patients demonstrated bilaterally functioning units preprocedure. Apart from the patient with misplaced ureteral stent, no patients underwent any diversion prior to definitive reconstruction. Twenty-two patients underwent laparoscopic ureteroneocystostomy with psoas hitch and 14 patients underwent ureteroneocystostomy only. Operative parameters and postoperative profile are listed in Table 2. A statistically significant difference was observed in operation duration. No statistical difference was observed in any other parameters between early and delayed repairs [Table 2]. Mean follow-up duration was 16.34 months (range five to 48 months). All patients in Group I and 14 patients in Group II completed at least six months follow-up. One patient in Group I complained of urinary infection during the follow-up period that was managed by oral antibiotics. The follow-up serum creatinine values were not statistically different (Group I 0.91 ± 0.13 mg/dl vs Group II 0.96 ± 0.24 mg/dl, P=0.33, Aspin Welch unequal variance t test). Satisfactory result in follow-up contrast imaging (no hydronephrosis and satisfactory drainage through the reconstructed unit) was appraised in all patients in Group I and Group II.
Table 1.
Demographic profile

Table 2.
Comparison of operative/postoperative profile Group I (early repair) vs Group II (delayed repair)

DISCUSSION
Lower ureteric strictures demand immediate attention to ensure unobstructed drainage of urine from the renal unit. Endourological reconstruction of these pathologies seldom suffices.[5] In the presence of a salvagable renal unit, uretereoneocystostomy with or without Boari flap remains the most desired option for restoration of ureteral continuity. Contrastingly, in iatrogenic lower ureteric injuries conventional practice entails attempting a temporising measure (ureteral stent or nephrostomy) primarily, deferring definitive reconstruction for an interval of six weeks.[2] Unfamiliarity of the surgical anatomy, difficulty in access, hemodynamic instability of the sufferer or altered renal profile has been reasoned for adopting this approach. Another popular concept favoring delayed repair is superior appraisal of the extent of the unhealthy tissue and thereby a better postreconstruction outcome can be achieved. Due to limited experience with laparoscopic exercise, the incisional approach remains popular for these reconstructions.
Unfortunately, this prolongs the recovery of the sufferer and adds to the morbidity incurred. We implemented two deviations from this established protocol in our practice. First, early reconstructions were attempted without resorting to temporary diversion. Secondly, all reconstructions were attempted via laparoscopic approach irrespective of the duration since the primary insult. More than half of our patient cohort underwent definitive repair significantly early (within seven days of the primary event). However, apart from operation duration, no statistically significant difference was perceived in any of the operative or postoperative parameters between early and delayed repairs. The long term outcome parameters (clinical profile, renal profile and appearance at follow-up imaging) were also comparable. All procedures could be successfully completed laparoscopically. Early repairs were more technically demanding as perceived by the significant difference in operative time but there were no difference in blood loss incurred or complications attributable to the definitive event. Thus, if the operator is sufficiently conversant with laparoscopic exercise, these repairs may be conveniently accomplished via this approach. The magnified image as obtained in laparoscopy helps in distinguishing healthy from unhealthy tissue and aids in satisfactory reconstruction. Additionally, better appreciation of tissue planes enables meticulous dissection with preservation of periureteral adventitia. A few prerequisites should be fulfilled prior to embarking on laparoscopic ureteric reconstruction. Preoperative retrograde pyelogram is an extremely valuable tool that should be performed immediately prior to these reconstructions. This can significantly influence the choice of corrective procedure. Preoperative cystoscopy rules out co-existent bladder pathologies that may negate these reconstructions. In sufferers complaining of voiding dysfunction, preoperative urodynamic assessment is worthwhile. In the presence of abnormal cystometry, reconstructions involving bladder flap should be avoided and an alternative approach for ureteric reconstruction such as incorporation of intestinal segments may be advisable. Few techniques have been suggested for achieving satisfactory ureterovesical rehabilitation via laparoscopic approach.[3,6–8] The key issues for these procedures are preservation of adequate vascular supply to the lower ureter and construction of a tension free anastomosis. The lower ureter should be freed of all surrounding adhesions. During ureteric adhesiolysis, limited usage of thermal energy in the vicinity of the ureter minimises the extent of ischemic endarteritis and renders a superior outcome. Unnecessary mobilisation of the upper ureter (part of ureter cranial to ipsilateral sacroiliac joint) raises the possibility of jeopardising the upper ureteral blood supply and should be strictly condemned. In ureteroneocystostomies, wide mobilisation of the bladder is crucial and the need for psoas hitch is dictated by the tension apprehensed during ureterovesical approximation. Longer ureteral defects that cannot be bridged by primary ureteroneocystostomy even after sufficient bladder mobilisation mandate construction of Boari flap ureteroneocystostomies.[9,10] Although we have performed laparoscopic Boari flap for ureteral reconstructions in both solitary and bilaterally functioning units, all these procedures were performed for noniatrogenic etiology, and hence the results are not included in this analysis. No antireflux procedures were practiced in our patient cohort. The decision for addition of an antireflux technique depends on the patient's age and does not render any additional benefit over refluxing reconstructions in adult male or females.[8] Construction of an antireflux technique also mandates additional ureteral mobilization to allow satisfactory tunneling and may be difficult in presence of long segment ureteral loss. Wide mobilization and tunneling may further jeopardize the already precarious blood supply to the ureter and herald an unsatisfactory outcome. No episodes of recurrent pyelonephritis or decline in renal profile were encountered in our patient cohort on long term follow-up. An important aspect of all these techniques that needs be emphasized is achievement of satisfactory placement of ureteric stent to bridge the anastomosis. A misplaced ureteric stent may disrupt the ureterovesical anastomosis and culminate in restenosis.[11] The upper end of the ureteral stent may be threaded over a preplaced guidewire under fluoroscopy monitoring and the lower end should be placed in the bladder under direct supervision. A postoperative skiagram should be evaluated to confirm satisfactory stent placement. The long term outcome following laparoscopic reconstruction is appreciable. No patients in either group revealed any deterioration in clinical or renal profile during follow-up period. On follow-up imaging, satisfactory results were obtained universally. This supports the efficacy of laparoscopic reconstruction of iatrogenic lower ureteric pathologies. The sufferer's hemodynamic profile or renal parameters may influence the timing of the definitive approach. All our subjects were hemodynamically stable with normal renal profile at presentation. Although we have obtained satisfactory results following laparoscopic ureteric reconstruction in patients with altered renal profile, these subjects were of noniatrogenic etiology, and hence these results have not been included in this interpretation. The primary benefit of immediate definitive repair is avoidance of the morbidity secondary to the temporizing nephrostomy. Additional advantages of minimally invasive access are enhanced cosmesis, early ambulation, improved pain tolerance and early return to work. On many occasions, the insults were incurred during laparoscopic procedures and the corrective procedure was successfully accomplished with a minimally invasive approach. This enabled preservation of body image. Laparoscopic approach for ureteral reconstructions demands technical expertise. Familiarity with pelvic anatomy, proficiency in laparoscopic tissue dissection and expertise in intracorporeal suturing are mandatory.
CONCLUSION
Laparoscopic reconstruction of lower ureteral strictures secondary to iatrogenic insults are feasible and in hemodynamically stable patients may be undertaken primarily without resorting to temporizing diversion. The immediate and long term outcome following early and delayed repair is comparable. Early repair albeit technically more demanding than delayed repairs, limits additional morbidity attributable to diversion and delayed repair. Limited additional morbidity is sustained due to the definitive restorative procedure.
ACKNOWLEDGMENT
Sooraj Rajsekaran Kartha contributed the statistical analysis for this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Simmons MN, Gill IS, Fergany AF, Kaouk JH, Desai MM. Technical modifications to laparoscopic Boari flap. Urology. 2007;69:175–80. doi: 10.1016/j.urology.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Jack McAninch, Santucci RA. Renal and ureteral trauma. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Campbell Walsh urology. 9th ed. philadelphia: elsiever; 2007. p. 1289. [Google Scholar]
- 3.Kaouk JH, Gill IS. Laparoscopic reconstructive urology. J Urol. 2003;170:1070–8. doi: 10.1097/01.ju.0000073207.06071.dc. [DOI] [PubMed] [Google Scholar]
- 4.Siedeman CA, Huckabay C, Smith KD, Permpongkosol S, Nadjafi-Semnani M, Lee BR, et al. Laparoscopic ureteral reimplantation: Technique and outcomes. J Urol. 2009;181:1742–6. doi: 10.1016/j.juro.2008.11.102. [DOI] [PubMed] [Google Scholar]
- 5.Razdan S, Silberstein IK, Bagley DH. Ureteroscopic endoureterotomy. BJU Int. 2005;95(Suppl 2):94–101. doi: 10.1111/j.1464-410X.2005.05207.x. [DOI] [PubMed] [Google Scholar]
- 6.Modi P, Gupta R, Rizvi SJ. Laparoscopic ureteroneocystostomy and psoas-hitch for post-hysterectomy ureterovaginal fistula. J Urol. 2008;180:615–7. doi: 10.1016/j.juro.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Gao J, Dong J, Xu A, Wang W, Shi L, Guo G, et al. Simplified technique for laparoscopic ureteroneocystostomy without ureteral nipple or submucosal tunneling. J Endourol. 2007;21:1505–8. doi: 10.1089/end.2007.0065. [DOI] [PubMed] [Google Scholar]
- 8.Symons S, Kurien A, Desai M. Laparoscopic ureteral reimplantation: A single centre experience with literature review. J Endourol. 2009;23:269–74. doi: 10.1089/end.2008.0266. [DOI] [PubMed] [Google Scholar]
- 9.Castillo OA, Litvak JP, Kerkebe M, Olivares R, Urena RD. Early experience with the laparoscopic Boari flap at a single institution. J Urol. 2005;173:862–5. doi: 10.1097/01.ju.0000152157.25984.ae. [DOI] [PubMed] [Google Scholar]
- 10.Fugita OE, Dinlenc C, Kavoussi L. The laparoscopic Boari flap. J Urol. 2001;166:51–3. [PubMed] [Google Scholar]
- 11.Abraham GP, Das K, George DP. Retroperitoneal migration of double J ureteral stent: An unusual occurrence. J Endourol. 2011;25:297–9. doi: 10.1089/end.2010.0281. [DOI] [PubMed] [Google Scholar]


