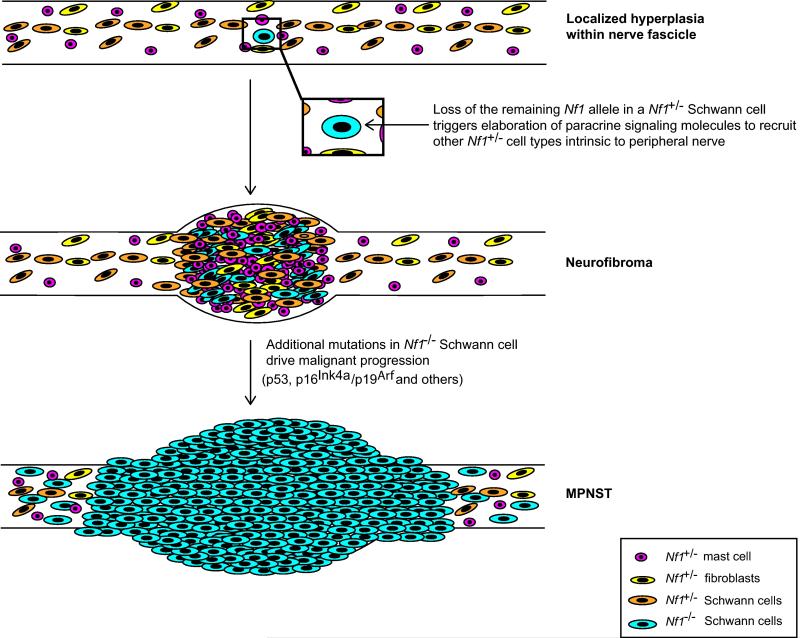
Fig. 7. Major events in the pathogenesis of a neurofibroma and its subsequent progression to become a MPNST.
Illustrated are the changes leading to neurofibroma and MPNST development in GEM models of NF1. Transient hyperplasia is often observed in the peripheral nerves of these animals prior to neurofibroma development; it is unclear whether an Nf1+/− background is sufficient to generate this phenotype or whether biallelic loss of Nf1 in Schwann cells must also occur. Neurofibroma development, however, does depend upon biallelic loss of Nf1 in Schwann cells or their less differentiated precursors. Loss of the remaining functional Nf1 allele in the Schwann cell lineage triggers elaboration of paracrine signaling molecules that recruit other Nf1+/− cell types (including mast cells, fibroblasts, and other Schwann cells) from the peripheral nerve to the nascent tumor site. As not all Nf1−/− Schwann cells generate neurofibromas, there are likely other as-yet-undetermined events that must occur to the Nf1−/− Schwann cell or its microenvironment to trigger tumor formation. Once the neurofibroma has been established, additional mutations in tumor suppressor genes such as TP53 and p16Ink4a/p19Arf in Nf1−/− Schwann cells drive malignant progression. Comparisons with human neurofibromas and MPNSTs indicate that most of these events are relevant to the pathogenesis of the human counterparts of these murine tumors. However, the relevance of some changes seen in the murine tumor models (e.g., the initial phase of intraneural hyperplasia) remains to be determined. It should also be noted that other events associated with malignant progression in human MPNSTs (e.g., suppression of Sox10 expression and upregulation of Sox9 expression, altered epigenetic modification or miRNA regulation programs) have not yet been thoroughly explored in genetically engineered mouse models of peripheral nerve sheath tumors.