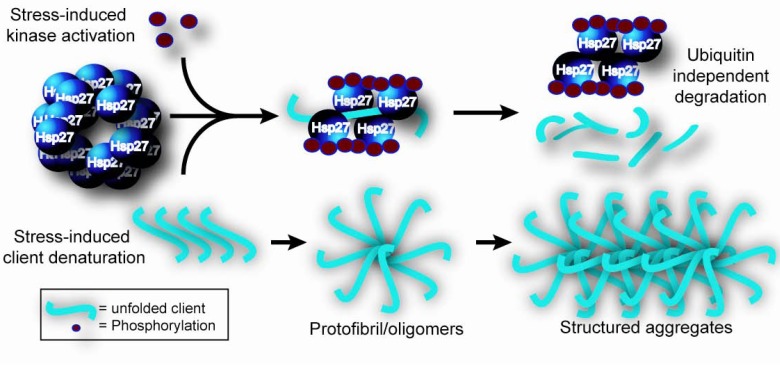
Fig. (1).
The necessity of phosphorylation-dependent Hsp27 function for preventing protein aggregation of unfolded intermediates. Large molecular weight Hsp27 multimers are constitutively present in the cell under basal conditions. Upon extracellular stress, the p38 MAP kinase pathway is activated, phosphorylating Hsp27 (red circles). Consequently, the Hsp27 multimeric complex disassembles into smaller complexes that can bind the denatured client and prevent its subsequent aggregation. The client can then be targeted for degradation in a mechanism that is independent of ubiquitin. Without Hsp27, folding intermediates of the client would be prone to aggregate.