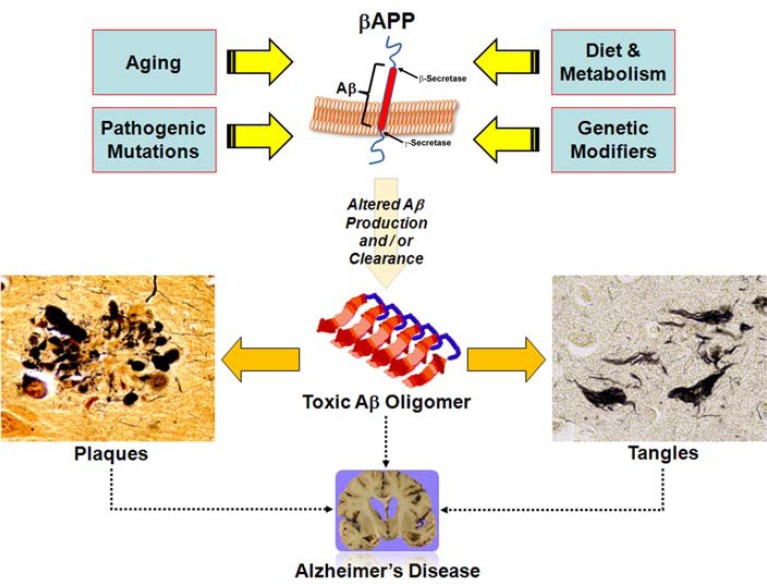
Fig. (2).
Factors Affecting Amyloid Accumulation and AD Pathology. In a broad sense, AD is characterized by neuronal degeneration, plaques and tangles. Strong evidence implicates the amyloid-β precursor protein (APP), the source of the amyloid-β peptide (Aβ), as the central player in the pathophysiology of the disease. Various factors (pathogenic mutations, genetic modifiers, diet and metabolism, and even the aging process itself) conspire so that the steady state levels of Aβ are extremely high in the AD brain. This can occur because of higher APP expression, increased APP metabolism (more β- and / or γ-secretase activity), decreased Aβ catabolism, faulty clearance of Aβ from the brain, or some combination of these processes. Elevated levels of Aβ, particularly aggregation prone species such as Aβ42, lead to an increase in the amount of higher order oligomeric forms of the peptide (made up of two or more Aβ molecules). These intermediates exist, at least transiently, in a toxic soluble form which probably exists both inside and outside the cell. There is evidence that oligomeric Aβ damages neurons, leads to neurofibrillary tangles and eventual cell death, and ultimately forms highly insoluble fibrils that eventually deposit as plaques in the brain parenchyma. Oligomeric Aβ also likely has other deleterious effects on neuronal function, only some of which have been characterized to date. Although AD is the best studied amyloidopathy, similar mechanisms (acting on proteins other than APP) may lie at the heart of many other neurodegenerative diseases. It is possible that knowledge of the common mechanisms contributing to these disease processes may lead to therapeutic approaches that are at least partially effective against neurodegeneration in general.