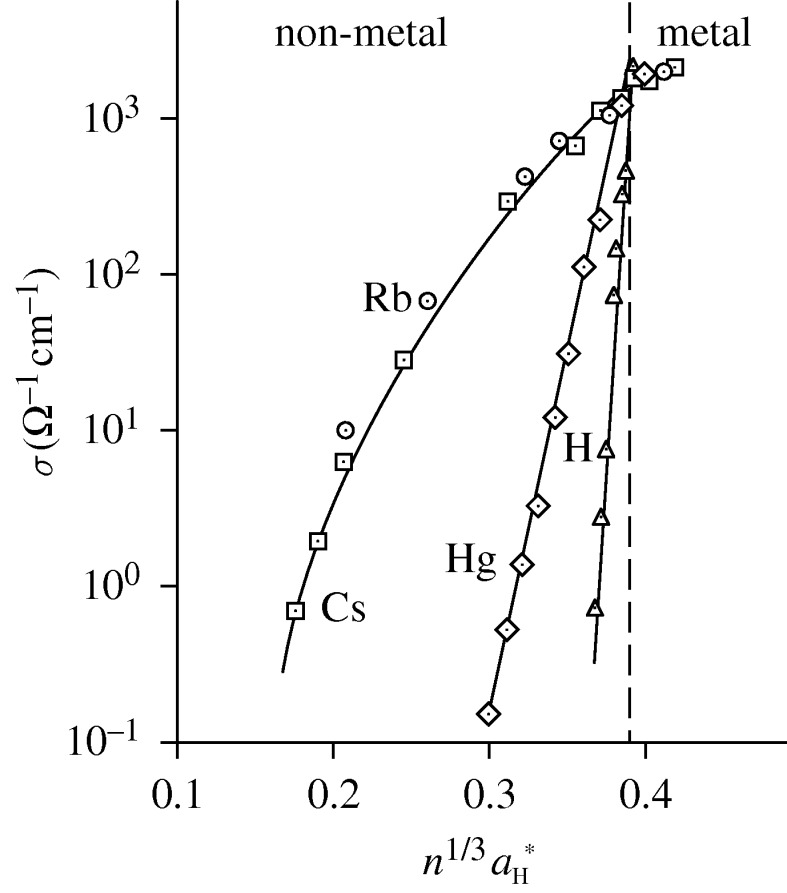
Figure 9.
The transition to the metallic state for high-temperature (T> 1000 K) fluid caesium, rubidium, mercury and hydrogen: the dependence of the electrical conductivity on the scaling parameter n1/3aH*. The dotted line drawn at n1/3aH* indicates the common metallization condition for these chemical elements. To the left of the metallization condition, we have the non-metallic form of the four elements (non-metallic fluids); to the right, we have the corresponding metallic state (the metallic fluids). Above the metallization threshold, we anticipate conduction based on the theory put forward by Ziman (1961).