Abstract
OBJECTIVE
Data on cardiac function in patients with nonalcoholic fatty liver disease (NAFLD) are limited and conflicting. We assessed whether NAFLD is associated with abnormalities in cardiac function in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
We studied 50 consecutive type 2 diabetic individuals without a history of ischemic heart disease, hepatic diseases, or excessive alcohol consumption, in whom NAFLD was diagnosed by ultrasonography. A tissue Doppler echocardiography with myocardial strain measurement was performed in all patients.
RESULTS
Thirty-two patients (64%) had NAFLD, and when compared with the other 18 patients, age, sex, BMI, waist circumference, hypertension, smoking, diabetes duration, microvascular complication status, and medication use were not significantly different. In addition, the left ventricular (LV) mass and volumes, ejection fraction, systemic vascular resistance, arterial elasticity, and compliance were also not different. NAFLD patients had lower e′ (8.2 ± 1.5 vs. 9.9 ± 1.9 cm/s, P < 0.005) tissue velocity, higher E-to-e′ ratio (7.90 ± 1.3 vs. 5.59 ± 1.1, P < 0.0001), a higher time constant of isovolumic relaxation (43.1 ± 10.1 vs. 33.2 ± 12.9 ms, P < 0.01), higher LV–end diastolic pressure (EDP) (16.5 ± 1.1 vs. 15.1 ± 1.0 mmHg, P < 0.0001), and higher LV EDP/end diastolic volume (0.20 ± 0.03 vs. 0.18 ± 0.02 mmHg, P < 0.05) than those without steatosis. Among the measurements of LV global longitudinal strain and strain rate, those with NAFLD also had higher E/global longitudinal diastolic strain rate during the early phase of diastole (E/SRE). All of these differences remained significant after adjustment for hypertension and other cardiometabolic risk factors.
CONCLUSIONS
Our data show that in patients with type 2 diabetes and NAFLD, even if the LV morphology and systolic function are preserved, early features of LV diastolic dysfunction may be detected.
The prevalence of obesity and type 2 diabetes has reached epidemic proportions worldwide (1–3). It is known that type 2 diabetes is associated with premature death and is an established risk factor for cardiovascular disease (CVD), particularly for ischemic heart disease and chronic heart failure (2,3). Abnormalities in cardiac structure and function in type 2 diabetic individuals may, however, develop even in the absence of ischemic heart disease or hypertension. These abnormalities are attributed to diabetic cardiomyopathy, in which the basic pathophysiologic mechanisms still remain poorly known (4,5). Diabetic cardiomyopathy may induce changes in cardiac structure such as myocardial hypertrophy, fibrosis, and fat droplet deposition. Early changes in cardiac function are also manifested as abnormal diastolic function that with time can lead to loss of contractile function (4–7).
In parallel, it is recognized that nonalcoholic fatty liver disease (NAFLD) is largely prevalent in subjects who are obese or have type 2 diabetes (8–10). Nowadays, growing evidence suggests that NAFLD is linked to increased risk of CVD events in nondiabetic and type 2 diabetic individuals (11). Several investigators have examined the association of NAFLD with markers of subclinical CVD (e.g., carotid artery intima-media thickness) or clinical CVD (11). Conversely, the information regarding abnormalities in cardiac function among NAFLD patients is limited and controversial. It has been shown that nondiabetic, normotensive patients with NAFLD have echocardiographic features of early left ventricular (LV) diastolic dysfunction as measured by tissue Doppler echocardiography (12,13) and impaired LV energy metabolism, as measured by cardiac 31P-magnetic resonance spectroscopy (MRS), compared with control subjects without steatosis (14). In a recent study involving type 2 diabetic men, Rijzewijk et al. (15) found that, compared with those with lower intrahepatic fat content, patients with higher intrahepatic fat content, as measured by 1H-MRS, had impaired myocardial perfusion and lower high-energy phosphates but similar values of LV function and morphology (as detected by cardiac magnetic resonance imaging [MRI]).
Since two-dimensional echocardiography using tissue Doppler imaging is the most simple and reliable imaging method to evaluate early, subclinical changes in LV function (4–7), we wanted to apply this technique to test whether subtle cardiac abnormalities could be detected in type 2 diabetic individuals with NAFLD in comparison with those without steatosis.
RESEARCH DESIGN AND METHODS
We studied 50 white type 2 diabetic outpatients (38 men; mean age 64 ± 5 years), who consecutively attended the diabetes clinic of Sacro Cuore Hospital of Negrar during a period of 18 months. We excluded 1) patients who had a prior history of ischemic heart and valvular disease, chronic heart failure, cirrhosis, or overt nephropathy; 2) patients with poor and unstable glycemic control, and 3) those who had known causes of chronic liver disease (i.e., alcohol- or drug-induced liver disease, hemochromatosis, or autoimmune or viral hepatitis). Additional exclusion criterion was the use of thiazolidinediones. All women were of postmenopausal status and did not take hormonal replacement therapy.
A 12-lead standard resting electrocardiogram, 24-h Holter monitoring, bicycle ergometry, and conventional Doppler echocardiography were performed in all patients to exclude the presence of silent myocardial ischemia or significant disturbances of sinus rhythm; no patients had any abnormal test results. Stress echocardiography during exercise or administration of pharmacological agents was not performed.
Of the 50 participants included in the study, 32 (64%) patients met the clinical criteria for a diagnosis of NAFLD (i.e., hepatic steatosis on ultrasound among persons who drank <20 g/day of ethanol and who did not have viral hepatitis, drug-induced liver disease, iron overload, or other known causes of liver disease) and 18 (36%) patients did not.
The local ethics committee approved the study. All participants gave written informed consent for participation in medical research.
Clinical and laboratory data
BMI was calculated by dividing weight in kilograms by height in meters squared. Waist circumference was measured midway between the lower-rib margin and the superior anterior iliac crest. Blood pressure was measured in duplicate by a physician with a mercury sphygmomanometer (at the right upper arm using an appropriate cuff size) after participants had been seated quietly for at least 5 min. In all participants, the presence of microvascular complications such as sensory neuropathy (by biothesiometer) and nephropathy (by serum creatinine and albuminuria measurements) were also recorded. Information on smoking and alcohol consumption was obtained from all participants by a validated questionnaire.
Blood samples were drawn in the morning after an overnight fast. Serum liver enzymes, ferritin, creatinine, and other biochemical blood measurements were determined by standard laboratory procedures (DAX 96; Bayer Diagnostic, Milan, Italy). LDL cholesterol was calculated using the Friedewald equation. HbA1c was measured by a high-performance liquid chromatography analyzer (HA-8140; Menarini Diagnostics, Florence, Italy); the upper limit of normal for the laboratory was 5.6%. All patients had negative hepatitis B and C viral markers. Albuminuria was measured by an immunonephelometric method on a morning spot urine sample and expressed as the albumin-to-creatinine ratio.
Heart rate variability analysis
Heart rate variability (HRV) analysis was performed measuring the following time-domain parameters of 24-h HRV that were automatically calculated during Holter monitoring (Mortara Rangoni, Bologna, Italy): the SD of normal-to-normal R-R intervals (SDNN), the root mean square of difference of successive R-R intervals (rMSSD), and the percentage of adjacent R-R intervals varying by >50 ms (pNN50). SDNN is thought to represent joint sympathetic and parasympathetic modulation of HRV, and rMSSD and pNN50 are specific for the parasympathetic limb (16).
Echocardiographic evaluation
Conventional echocardiography was used to measure LV diameters, wall thickness, and mass according to standard criteria (17). LV end diastolic (EDV) and end systolic volumes and ejection fraction at rest were measured at the apical two-chamber and four-chamber views (by modified Simpson rule) (17). Left atrial maximal volume was measured at the end of LV systole from the apical two-chamber and four-chamber views (by modified Simpson rule) (17). Measurements were indexed to body surface area when appropriate. Pulsed-wave Doppler was used to measure transmitral peak early diastolic velocity (E), peak late diastolic velocity (A), and E-wave deceleration time (Dte). Isovolumetric relaxation time (IVRT) was also calculated (18). Each value was obtained from the average of three measurements.
Tissue Doppler imaging
Pulsed-wave tissue Doppler echocardiography of the septal and lateral mitral annulus was used to measure the early (e′) and late (a′) annular diastolic and systolic (s′) tissue velocities, and the mean values of septal and lateral annulus measurements were used for analysis (19,20). The e′ tissue velocity is relatively preload independent and correlates inversely with the time constant of isovolumic relaxation (τ), which is derived from the following formula: τ = (14.70 – 100 e′) / 0.15 (20,21). LV end diastolic pressure (EDP) was estimated as follows: LV EDP = 11.96 + 0.596 ⋅ E-to-e′ ratio (20). The time interval between the QRS complex and the onset of mitral E-wave velocity was subtracted from the time interval between the QRS complex and e′ onset to derive TE − e′, which strongly depends on the time constant of LV relaxation and minimal pressure. The ratio of IVRT to TE−e′ was then calculated; this ratio provides incremental information as to the E-to-e′ ratio on LV filling pressure in subjects with normal ejection fraction and E-to-e′ ratio between 8 and 15 (22).
Myocardial deformation measurements were also performed off-line in patients with adequate apical windows (n = 45) with the use of a standard EchoPac PC workstation application for two-dimensional strain analysis. Global longitudinal strain and strain rate curves were obtained including all six LV myocardial segments from four-chamber, two-chamber, and long-axis apical views (23). The average values of peak systolic longitudinal strain and peak systolic strain rate from the three apical views were calculated as global longitudinal strain (LSSYS) and global strain rate (SRSYS), respectively. Similarly, the global diastolic strain rate during the early (SRE) and late (SRL) phase of diastole was also calculated. The ratio of transmitral E-wave velocity to SRE as an index of LV filling pressure was calculated as previously proposed (24). Standard echocardiographic views were obtained using frequency, depth, and sector width adjusted for frame-rate optimization (between 60 and 100 frames per second). Tissue Doppler imaging was performed in all patients by a single experienced cardiologist, who was blinded to NAFLD details of participants. Eighteen tissue Doppler imaging signals were remeasured by the same observer; the mean ± SD absolute differences in tissue velocities within the same observer were 0.10 ± 0.02 cm/s for s′ velocity, 0.19 ± 0.17 cm/s for e′ velocity, and 0.23 ± 0.20 cm/s for a′ velocity, respectively (P = not significant for all differences). Eighteen tissue Doppler imaging signals were also remeasured by a second observer; the mean absolute differences in tissue velocities between the two observers were 0.11 ± 0.09 cm/s for s′ velocity, 0.30 ± 0.25 cm/s for e′ velocity, and 0.35 ± 0.28 cm/s for a′ velocity (P = not significant for all differences). No significant differences were found in the intraobserver and interobserver variabilities for global longitudinal strain (0.79 ± 0.60 and 1.07 ± 0.80%), SRSYS (0.08 ± 0.04 and 0.11 ± 0.07 s−1), and SRE (0.08 ± 0.06 and 0.12 ± 0.08 s−1).
Vascular function
Effective arterial elasticity was estimated as end-systolic pressure divided by stroke volume (25). End-systolic pressure was estimated as systolic pressure ×0.9 (25,26). Systemic arterial compliance was estimated by the stroke volume–to–pulse pressure ratio and systemic vascular resistance index by mean arterial pressure divided by cardiac index ×80 (27).
Hepatic and carotid ultrasonography
Hepatic ultrasonography was performed in all patients by a single experienced radiologist, who was blinded to the participants’ details. Hepatic steatosis was diagnosed on the basis of characteristic sonographic features, i.e., evidence of diffuse hyperechogenicity of the liver relative to the kidneys, ultrasound beam attenuation, and poor visualization of intrahepatic vessel borders and diaphragm (28). It is known that ultrasonography has a good sensitivity and specificity for detecting moderate and severe hepatic steatosis (∼90–95%), but its sensitivity is reduced when the hepatic fat infiltration upon liver biopsy is <30% (8–10,28). A semiquantitative ultrasonographic scoring for the degree of steatosis (absent, mild, moderate, and severe) was also performed. The degree of steatosis was assessed by the fall in echo amplitude with depth (rate of posterior beam attenuation), increasing discrepancy of echo amplitude between liver and kidney, and loss of echoes from the walls of the portal veins (28). The reproducibility of steatosis scores provided by our single blinded radiologist was very good (intraobserver agreement of ∼98%). The presence of stenosis ≥30% at the level of either internal or common carotid arteries was diagnosed by echo-Doppler scanning, which was performed by a single specialist physician, who was blind to subjects’ characteristics.
Statistical analysis
Data are presented as means ± SD or frequencies. Differences in clinical characteristics, metabolic variables, and echocardiographic parameters among participants stratified by NAFLD status were tested with one-way ANOVA models with Bonferroni post hoc correction test for normally distributed variables and Kruskal-Wallis test for non–normally distributed variables; the χ2 test was used to test differences in categorical variables between the groups. ANCOVA models included HbA1c, triglycerides, and hypertension (defined as blood pressure ≥140/90 mmHg or drug treatment) as covariates to statistically control for baseline differences in these variables. A multivariable logistic regression analysis was also used to identify the factors independently associated with an increased E-to-e′ ratio (categorized according to its median value, i.e., 7), which was included as the dependent variable. The covariates for the multivariable regression analysis were chosen as potential confounding factors based on their significance in univariate analysis (i.e., triglycerides and HbA1c) or based on their biological plausibility (age, sex, and hypertension). P values <0.05 were considered statistically significant.
RESULTS
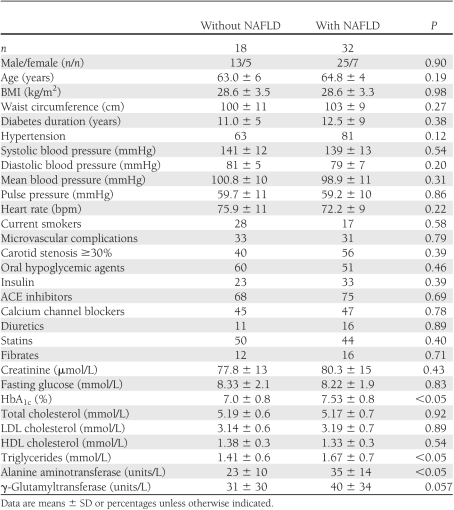
Clinical and biochemical characteristics of participants stratified by NAFLD status are summarized in Table 1. NAFLD patients had higher plasma triglycerides and HbA1c than those without steatosis, although the glycemic control of participants was fairly good (mean HbA1c 7.3%). As expected, they also had higher serum liver enzymes, although the vast majority of our NAFLD patients, i.e., ∼90%, had serum alanine aminotransferase and γ-glutamyltransferase concentrations within the reference ranges (normal ranges for alanine aminotransferase and γ-glutamyltransferase, in our laboratory, were 10–40 units/L for women and 10–50 units/L for men, respectively). Age, sex, BMI, waist circumference, smoking status, heart rate, systolic/diastolic blood pressure, LDL cholesterol, HDL cholesterol, fasting glucose, duration of diabetes, microvascular complication status (abnormal albuminuria and sensory neuropathy), frequency of carotid stenosis ≥30%, and treatments for hypertension, dyslipidemia, and diabetes did not differ between the groups.
Table 1.
Clinical and biochemical characteristics of type 2 diabetic patients grouped by NAFLD status
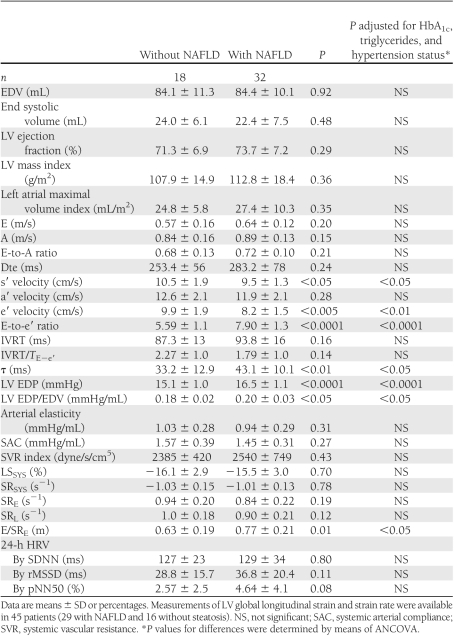
Table 2 shows the echocardiographic characteristics of participants grouped according to NAFLD status. NAFLD patients had lower e′ and s′ tissue velocities, higher E-to-e′ ratio, higher LV EDP, higher τ, and a higher LV EDP-to-EDV ratio than those without NAFLD. Among the measurements of global LV strain and strain rate, those with NAFLD also had higher E/SRE and tendentially lower SRE and SRL. No significant differences were found in LV EDVs and end systolic volumes and ejection fraction, indexed LV mass, indexed left atrial volume, E and A transmitral wave velocities, E-to-A ratio, Dte, IVRT, systemic arterial elasticity, compliance, vascular resistance, and 24-h HRV parameters between the groups.
Table 2.
Echocardiographic and hemodynamic characteristics of type 2 diabetic patients grouped by NAFLD status
When patients were categorized into two groups according to an E-to-e′ ratio >8 (i.e., if ≤8, left atrial pressure was considered normal), none of those without steatosis had an E-to-e′ ratio >8, whereas 43.7% of those with NAFLD had an E-to-e′ ratio >8 (P < 0.0001 for the difference between the groups). No patients had an E-to-e′ ratio >15.
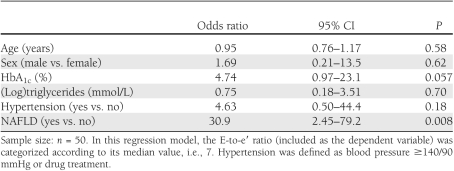
Notably, as shown in Table 2, the differences in echocardiographic characteristics, including also the E-to-e′ ratio, remained statistically significant after adjustment for HbA1c, triglycerides, and hypertension status (by ANCOVA). Similarly, in multivariate logistic regression analysis (Table 3), NAFLD was the strongest correlate of increased E-to-e′ ratio after adjustment for age, sex, triglycerides, HbA1c, and hypertension. HbA1c and hypertension also tended to be associated with increased E-to-e′ ratio but did not reach statistical significance. Almost identical results were observed when we performed a multivariable linear regression model, in which the E-to-e′ ratio was included as a continuous variable (data not shown). However, the results of these regression models should be interpreted with caution, given the relatively low number of patients.
Table 3.
Multivariable logistic regression analysis: independent predictors of increased E-to-e′ ratio in patients with type 2 diabetes
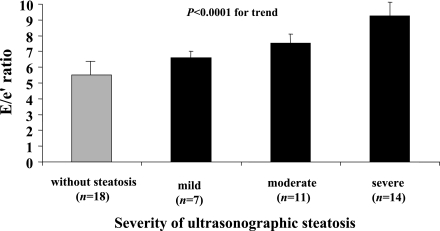
As shown in Fig. 1, when participants were categorized into groups according to the ultrasonographic severity of steatosis, the E-to-e′ ratio gradually increased across the groups (P < 0.0001 for the trend). The positive, graded relationship between the E-to-e′ ratio and steatosis scores remained significant after adjusting for age, sex, triglycerides, HbA1c, and hypertension (P < 0.001 by ANCOVA). Supplementary Table 1, available in the Supplementary Data, shows all of the echocardiographic parameters of participants stratified by different levels of hepatic echogenicity.
Figure 1.
Relationship between the severity of ultrasonographic hepatic steatosis and the E-to-e′ ratio as measured by tissue Doppler imaging in patients with type 2 diabetes. Data are expressed as means ± SD.
CONCLUSIONS
We have shown for the first time that in type 2 diabetic patients with NAFLD, features of early, subclinical LV diastolic dysfunction may be detected, as measured by two-dimensional echocardiography using tissue Doppler imaging, which is currently the preferred diagnostic approach for evaluating subclinical changes in LV function (4–7). Additionally, because diastolic dysfunction was not detected by conventional echocardiography, our results provide further evidence that tissue Doppler imaging is more accurate and sensitive than conventional echocardiography for detecting early alterations in LV diastolic function in type 2 diabetic patients (4–7).
LV dysfunction in patients with NAFLD
Our patients with NAFLD were characterized by significantly lower e′ tissue velocity, higher E-to-e′ ratio, higher LV EDP, higher τ, and higher LV EDP/EDV (an index of diastolic stiffness) compared with their counterparts without steatosis. Measurements of LV global longitudinal strain and strain rate by speckle tracking analyses further confirmed these findings (i.e., NAFLD patients had significantly higher E/SRE and tended to have lower SRE and SRL). These data suggest that NAFLD may contribute to impairments of both active and passive LV diastolic proprieties that are probably additive to the defects already present in type 2 diabetes. Moreover, our NAFLD patients also had a slight but significant reduction in s′ tissue velocity compared with those without steatosis, implying a potential adverse impact of NAFLD also on systolic function. Since no significant differences were found between patients with and without NAFLD in afterload conditions either in terms of “steady” (i.e., mean blood pressure) or in terms of “pulsatile” components of the arterial load (i.e., pulse pressure and arterial compliance and elasticity), our data suggest that the alterations in LV diastolic function were not attributable to different afterload conditions.
Notably, the above-described echocardiographic features of early LV diastolic dysfunction that we observed in NAFLD patients were independent of several potential confounders, including age, sex, diabetes duration, HbA1c, medication use, hypertension, and other cardiometabolic risk factors. Moreover, no participants were treated with pioglitazone, which has been shown to lower intrahepatic fat content (29) as well as to reduce intramyocardial fat content and improve some measures of LV diastolic function in type 2 diabetic subjects (30).
Comparison with previous studies
Data on cardiac function in patients with NAFLD are limited and conflicting. Our results extend for the first time to patients with type 2 diabetes (through our study’s use of more contemporary echocardiographic measures and strain analyses). Previous observations demonstrated that nondiabetic subjects with NAFLD had early alterations in LV diastolic function, as detected by tissue Doppler imaging, compared with control subjects without steatosis (12,13). Conversely, Perseghin et al. (14) showed that nondiabetic men with higher intrahepatic fat content, as measured by 1H-MRS, had significant alterations in myocardial high-energy phosphate metabolism (i.e., lower myocardial phosphocreatinine-to-ATP ratio) compared with those with lower intrahepatic fat content. However, these alterations of myocardial energy metabolism were detected despite similar values of LV morphology and function (by cardiac MRI) (14). Similarly, in a recent study involving 61 type 2 diabetic men without inducible myocardial ischemia, Rijzewijk et al. (15) found that compared with those with lower intrahepatic fat content, patients with higher intrahepatic fat content had decreased myocardial perfusion and lower myocardial phosphocreatinine-to-ATP ratio but similar values of LV function and morphology (by cardiac MRI). Finally, in a study involving 55 type 2 diabetic men with known ischemic heart disease, Lautamäki et al. (31) found that patients with higher intrahepatic fat content had reduced coronary functional capacity; however, measurements of LV function and morphology were not performed in that study.
Possible mechanistic explanations
Plausible explanations for our findings could be that the association between NAFLD and diastolic dysfunction simply reflects the coexistence of LV hypertrophy (hypertensive heart disease) and, possibly, subclinical myocardial ischemia. However, we do not think this is the case. In fact, we did not find any significant differences in the frequency and drug treatment of hypertension, the total vascular load, or the LV mass between the groups; moreover, the association between NAFLD and diastolic dysfunction also remained significant after adjustment for hypertension and other cardiometabolic risk factors. We think the presence of subclinical myocardial ischemia, even if it could not be fully ruled out (i.e., a stress echocardiography was not performed), should be considered a remote possibility on the basis of patients’ clinical history, 24-h Holter monitoring, and standard bicycle ergometry. In addition, it should also be noted that subclinical diabetic cardiomyopathy is characterized by impaired myocardial blood flow at rest, which is not significantly correlated with early alterations in diastolic and systolic functions as revealed by tissue Doppler imaging and myocardial deformation analysis (32).
Another plausible explanation for our findings could be that NAFLD is a marker of ectopic fat accumulation in different organs, including the myocardium (i.e., myocardial steatosis). Rijzewijk et al. (33) showed that intramyocardial fat content, as detected by 1H-MRS, was significantly higher in uncomplicated type 2 diabetic men than in nondiabetic control subjects and was associated with impaired LV diastolic function as detected by cardiac MRI. Interestingly, the same authors also noted a significant, positive association between intramyocardial and intrahepatic fat contents after adjustment for diabetic state. Ng et al. (34) confirmed that higher intramyocardial fat content was associated with more pronounced impairment of biventricular strain and strain rate in type 2 diabetic men. However, measurements of intrahepatic fat content were not performed in that study. In contrast, McGavock et al. (35) showed that although intramyocardial fat accumulation was higher in type 2 diabetic patients than in nondiabetic control subjects, there was not a significant association between myocardial steatosis and LV ejection fraction or early diastolic filling dynamics.
Interestingly, emerging evidence also suggests that NAFLD, especially in its necroinflammatory form (nonalcoholic steatohepatitis), not only is a marker of CVD and cardiac function abnormalities but also might be involved in their pathogenesis, possibly through the systemic release of several pathogenic mediators from the steatotic and inflamed liver (e.g., increased C-reactive protein, interleukin-6, tumor necrosis factor-α, and other inflammatory cytokines) or through the contribution of NAFLD itself to systemic/hepatic insulin resistance and postprandial lipemia (10,11,36).
Collectively, these findings suggest the presence of complex and intertwined interrelationships between NAFLD, myocardial steatosis, and diastolic dysfunction. Further research is needed to elucidate the mechanisms by which NAFLD may contribute to the development of diastolic dysfunction.
Study limitations
The most important limitations of the study are the relatively small number of patients and its cross-sectional design, which precludes the establishment of causal and temporal relationships between NAFLD and diastolic dysfunction. Moreover, invasive measurements of LV filling pressure were not performed in this study. However, when compared with invasive reference methods, tissue Doppler imaging has been shown to accurately estimate LV filling pressure in patients with preserved systolic function (18). Finally, the diagnosis of NAFLD was based on ultrasonography and the exclusion of other known causes of liver disease but was not confirmed by liver biopsy. It is known that none of the radiological imaging techniques (ultrasound, computed tomography, or MRI) can distinguish between simple steatosis and more severe forms of NAFLD (i.e., nonalcoholic steatohepatitis) and that only liver biopsy can assess the severity of damage and the prognosis (8–10,28). However, liver biopsy would be unacceptable to perform in our patients, who had normal or only mildly elevated serum liver enzymes. Conversely, ultrasonography is by far the most common way of diagnosing NAFLD in clinical practice and has good sensitivity and specificity in detecting moderate and severe hepatic steatosis. Indeed, it has been reported that the presence of >30% fat on liver biopsy is optimal for ultrasound detection of steatosis, whereas ultrasonography is not totally sensitive, particularly when hepatic fat infiltration is <30% (8–10,28). Thus, although some nondifferential misclassification of NAFLD on the basis of ultrasonography is likely (i.e., some of the diabetic control patients could have underlying NAFLD despite normal serum liver enzymes and negative ultrasonography examination), this limitation would serve to attenuate the magnitude of our effect measures toward null; thus, our results can probably be considered conservative estimates of the association between NAFLD and LV diastolic dysfunction.
In summary, this study demonstrated a significant association between NAFLD and early LV diastolic dysfunction, independent of hypertension and several other potential confounders, in patients with well-controlled type 2 diabetes, without a history of ischemic heart disease, and with normal systolic function. Further studies are needed to examine the reproducibility of our results and to further elucidate the underlying mechanisms that link diastolic dysfunction and NAFLD in type 2 diabetes.
Supplementary Material
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
S.B. researched data and contributed to discussion. G.P. contributed to discussion and reviewed and edited the manuscript. G.M., G.C., and L.B. researched data and reviewed and edited the manuscript. G.Z. and E.B. contributed to discussion and reviewed and edited the manuscript. G.T. researched data, wrote the manuscript, and is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-1820/-/DC1.
References
- 1.Finucane MM, Stevens GA, Cowan MJ, et al. ; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet 2011;377:557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seshasai SR, Kaptoge S, Thompson A, et al. ; Emerging Risk Factors Collaboration Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association Standards of medical care in diabetes—2011. Diabetes Care 2011;34(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 2004;25:543–567 [DOI] [PubMed] [Google Scholar]
- 5.Maya L, Villarreal FJ. Diagnostic approaches for diabetic cardiomyopathy and myocardial fibrosis. J Mol Cell Cardiol 2010;48:524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang ZY, Yuda S, Anderson V, Short L, Case C, Marwick TH. Echocardiographic detection of early diabetic myocardial disease. J Am Coll Cardiol 2003;41:611–617 [DOI] [PubMed] [Google Scholar]
- 7.Galderisi M. Diastolic dysfunction and diabetic cardiomyopathy: evaluation by Doppler echocardiography. J Am Coll Cardiol 2006;48:1548–1551 [DOI] [PubMed] [Google Scholar]
- 8.de Alwis NMW, Day CP. Non-alcoholic fatty liver disease: the mist gradually clears. J Hepatol 2008;48(Suppl. 1):S104–S112 [DOI] [PubMed] [Google Scholar]
- 9.Marchesini G, Moscatiello S, Di Domizio S, Forlani G. Obesity-associated liver disease. J Clin Endocrinol Metab 2008;93(Suppl. 1):S74–S80 [DOI] [PubMed] [Google Scholar]
- 10.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology 2009;49:306–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363:1341–1350 [DOI] [PubMed] [Google Scholar]
- 12.Goland S, Shimoni S, Zornitzki T, et al. Cardiac abnormalities as a new manifestation of nonalcoholic fatty liver disease: echocardiographic and tissue Doppler imaging assessment. J Clin Gastroenterol 2006;40:949–955 [DOI] [PubMed] [Google Scholar]
- 13.Fotbolcu H, Yakar T, Duman D, et al. Impairment of the left ventricular systolic and diastolic function in patients with non-alcoholic fatty liver disease. Cardiol J 2010;17:457–463 [PubMed] [Google Scholar]
- 14.Perseghin G, Lattuada G, De Cobelli F, et al. Increased mediastinal fat and impaired left ventricular energy metabolism in young men with newly found fatty liver. Hepatology 2008;47:51–58 [DOI] [PubMed] [Google Scholar]
- 15.Rijzewijk LJ, Jonker JT, van der Meer RW, et al. Effects of hepatic triglyceride content on myocardial metabolism in type 2 diabetes. J Am Coll Cardiol 2010;56:225–233 [DOI] [PubMed] [Google Scholar]
- 16.Pop-Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care 2010;33:434–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, et al. ; American Society of Echocardiography’s Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology Recommendations for chamber quantification. Eur J Echocardiogr 2006;7:79–108 [DOI] [PubMed] [Google Scholar]
- 18.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009;22:107–133 [DOI] [PubMed] [Google Scholar]
- 19.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 1997;30:1527–1533 [DOI] [PubMed] [Google Scholar]
- 20.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 2000;102:1788–1794 [DOI] [PubMed] [Google Scholar]
- 21.Sohn DW, Chai IH, Lee DJ, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 1997;30:474–480 [DOI] [PubMed] [Google Scholar]
- 22.Rivas-Gotz C, Khoury DS, Manolios M, Rao L, Kopelen HA, Nagueh SF. Time interval between onset of mitral inflow and onset of early diastolic velocity by tissue Doppler: a novel index of left ventricular relaxation: experimental studies and clinical application. J Am Coll Cardiol 2003;42:1463–1470 [DOI] [PubMed] [Google Scholar]
- 23.Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr 2004;17:630–633 [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Khoury DS, Thohan V, Torre-Amione G, Nagueh SF. Global diastolic strain rate for the assessment of left ventricular relaxation and filling pressures. Circulation 2007;115:1376–1383 [DOI] [PubMed] [Google Scholar]
- 25.Kelly RP, Ting C-T, Yang T-M, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation 1992;86:513–521 [DOI] [PubMed] [Google Scholar]
- 26.Chen CH, Fetics B, Nevo E, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol 2001;38:2028–2034 [DOI] [PubMed] [Google Scholar]
- 27.Chemla D, Hébert JL, Coirault C, et al. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol 1998;274:H500–H505 [DOI] [PubMed] [Google Scholar]
- 28.Schwenzer NF, Springer F, Schraml C, Stefan N, Machann J, Schick F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J Hepatol 2009;51:433–445 [DOI] [PubMed] [Google Scholar]
- 29.Targher G, Bellis A, Fornengo P, et al. Prevention and treatment of nonalcoholic fatty liver disease. Dig Liver Dis 2010;42:331–340 [DOI] [PubMed] [Google Scholar]
- 30.van der Meer RW, Rijzewijk LJ, de Jong HW, et al. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation 2009;119:2069–2077 [DOI] [PubMed] [Google Scholar]
- 31.Lautamäki R, Borra R, Iozzo P, et al. Liver steatosis coexists with myocardial insulin resistance and coronary dysfunction in patients with type 2 diabetes. Am J Physiol Endocrinol Metab 2006;291:E282–E290 [DOI] [PubMed] [Google Scholar]
- 32.Moir S, Hanekom L, Fang Z-Y, et al. Relationship between myocardial perfusion and dysfunction in diabetic cardiomyopathy: a study of quantitative contrast echocardiography and strain rate imaging. Heart 2006;92:1414–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rijzewijk LJ, van der Meer RW, Smit JW, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 2008;52:1793–1799 [DOI] [PubMed] [Google Scholar]
- 34.Ng AC, Delgado V, Bertini M, et al. Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation 2010;122:2538–2544 [DOI] [PubMed] [Google Scholar]
- 35.McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation 2007;116:1170–1175 [DOI] [PubMed] [Google Scholar]
- 36.Stefan N, Kantartzis K, Häring HU. Causes and metabolic consequences of fatty liver. Endocr Rev 2008;29:939–960 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.