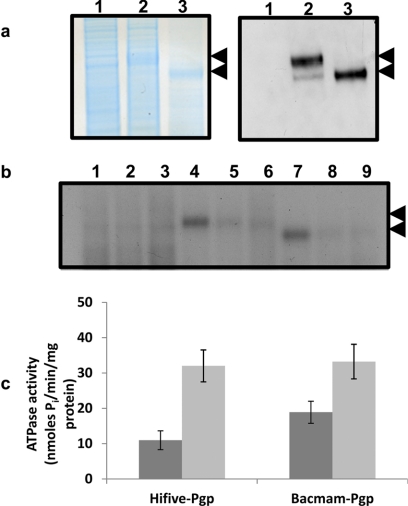
Fig. 2.
Crude membranes of BacMam-Pgp transduced HeLa cells overexpress functional Pgp. a, colloidal blue stain (left) and Western blot analysis (right) of crude membranes (10 μg protein for colloidal blue and 2 μg protein for Western blots) isolated from control HeLa cells (lane 1), HeLa cells transduced with BacMam Pgp (lane 2), and High-Five insect cells expressing Pgp (lane 3). Immunoblotting with C219, a Pgp-specific antibody, was performed as described under Materials and Methods. The arrows represent glycosylated (upper) or nonglycosylated (lower) forms of Pgp. b, Pgp expressed in BacMam-Pgp-transduced HeLa cells can be photolabeled with [125I]IAAP. Crude membranes (500 μg protein/ml) from control HeLa (lanes 1–3), Pgp-expressing HeLa (lanes 4–6), or High-Five insect cells expressing Pgp (lanes 7–9) cells were incubated for 5 min at room temperature in the absence (lanes 1, 4, and 7) or presence of 20 μM cyclosporine A (lanes 2, 5, and 8) or 10 μM tariquidar (lanes 3, 6, and 9) in 50 mM Tris-HCl (pH 7.5) and 3 to 6 nM [125I]IAAP (2200 Ci/mmol) was added. The samples were then photo-cross-linked with [125I]IAAP as described under Materials and Methods. The autoradiogram from a representative experiment is shown, and the arrow represents the position of glycosylated and unglycosylated forms of Pgp. A representative experiment from three independent experiments is shown here. c, Pgp expressed in crude membranes of BacMam-transduced HeLa cells exhibits substrate (verapamil)-stimulated ATPase activity. Crude membranes (100 μg protein/ml) from Pgp-expressing High-Five or HeLa cells were incubated at 37°C in the absence (dark gray) or presence (light gray) of 30 μM verapamil in the presence and absence of 0.3 mM sodium orthovanadate in ATPase assay buffer for 5 min, and the vanadate-sensitive ATPase activity of Pgp was determined as described under Materials and Methods. The histograms represent the ATPase activity (mean values ± S.D.) from three independent experiments.