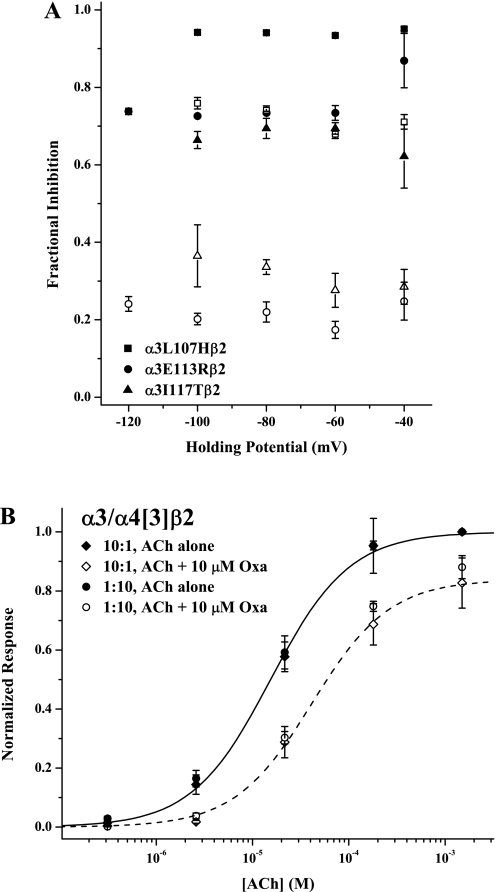
Fig. 7.
Oxantel inhibition of α3 mutants is noncompetitive and independent of subunit stoichiometry. A, the fractional degree of Oxa inhibition [(IACh − IACh+Oxa)/ IACh] is plotted versus holding potential for three point mutants (α3L107Hβ2, squares; α3E113Rβ2, circles; α3I117Tβ2, triangles). In all cases, the filled and open symbols represent the lower (20 μM) and higher (100 μM) ACh concentrations, respectively. Control and Oxa coapplied responses at each ACh concentration were recorded on the same set of oocytes; the Oxa concentration was 10 μM for α3E113Rβ2 and α3I117Tβ2 and 50 μM for α3L107Hβ2. Symbols represent means (± S.E.M.) for n = 3 or 4; in some cases, error bars are smaller than the symbol. B, the effect of subunit stoichiometry on Oxa inhibition of the triple mutant α3/α4[5]β2 is shown. Responses evoked by ACh alone are shown as ♦ (10:1 RNA injection ratio) and ● (1:10), whereas responses to ACh + 10 μM Oxa are given as ♢ (10:1) and ○ (1:10). All currents were normalized to the response to 1.5 mM ACh alone; values plotted are means ± S.E.M. For clarity, fits of the Hill equation to only the 10:1 ratio data are shown, with the solid curve for the ACh alone responses and the dashed curve for the Oxa inhibition case. The parameters of the Oxa inhibition, not reported elsewhere, were EC50, 42 ± 13 μM; nH, 1.08 ± 0.29; Emax, 0.84 ± 0.07 (10:1 ratio) and EC50, 40 ± 5 μM; nH, 1.09 ± 0.11; Emax, 0.90 ± 0.03 (1:10 ratio). The parameters for the control ACh responses were EC50, 14 ± 1 μM; nH, 1.01 ± 0.09 (10:1 ratio) and EC50, 15 ± 2 μM; nH, 1.06 ± 0.17 (1:10 ratio). The replicates were n = 5 for each experiment.