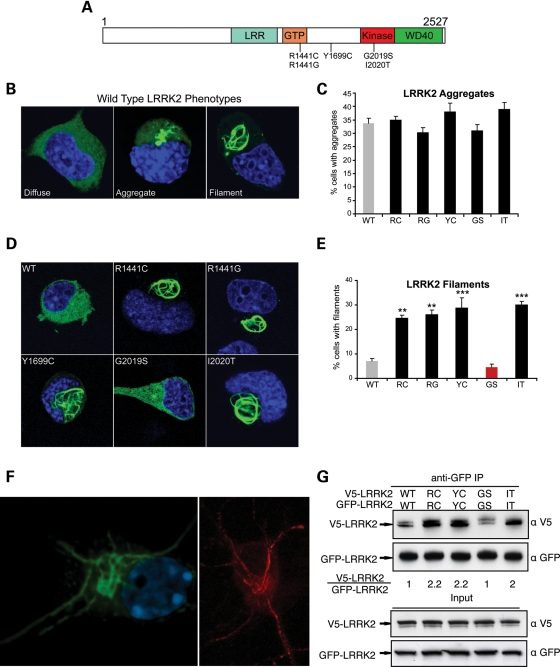
Figure 1.
Multiple pathogenic mutations enhance LRRK2 oligomerization and filament formation in a kinase-dependent manner. (A) Domain structure and Parkinson's disease mutations of LRRK2. LRR, leucine-rich repeat; GTP, GTPase domain (also called Roc domain, Ras of complex proteins). Five PD-causing missense mutations are shown. (B–E) The formation of LRRK2 filaments is enhanced by multiple PD mutations. Cath.a-differentiated (CAD) cells were transfected with WT (B) or PD mutant forms (D; R1441C, R1441G, Y1699C, G2019S, I2020T) of GFP-tagged LRRK2. WT-LRRK2 adopted either a diffuse, aggregated or filamentous pattern of subcellular localization (B; labeled using anti-GFP antibody). The frequency of cells bearing LRRK2 aggregates or filaments was quantified 48 h after transfection (C and E). Multiple PD mutations increased the percentage of cells with LRRK2 filaments (labeled using anti-GFP antibody). Data are means ± SE of four to five independent experiments (**P< 0.01, ***P< 0.001, n.s., non-significant; ANOVA with Tukey's test). (F) Expression of LRRK2 in neuronal processes. Yellow fluorescent protein (YFP)-LRRK2-Y1699C was transfected into primary cortical neurons and imaged by confocal microscopy (left image, YFP fluorescence shown in green). A similar pattern of filament formation was observed in neurites in primary neurons transfected with untagged LRRK2-I2020T (right image, labeled using an anti-LRRK2 antibody shown in red). (G) WT LRRK2 oligomerizes and multiple LRRK2 PD mutations enhance its oligomerization. V5-LRRK2 was co-expressed in CAD cells with GFP or GFP-LRRK2. Lysates were immunoprecipitated with anti-GFP 48 h after transfection, and the immunoprecipitates were analyzed with anti-V5 and anti-GFP immunoblots. The oligomeric state of LRRK2 is shown as a relative ratio of co-purified V5-LRRK2 to GFP-LRRK2, normalized to the WT-LRRK2 ratio.