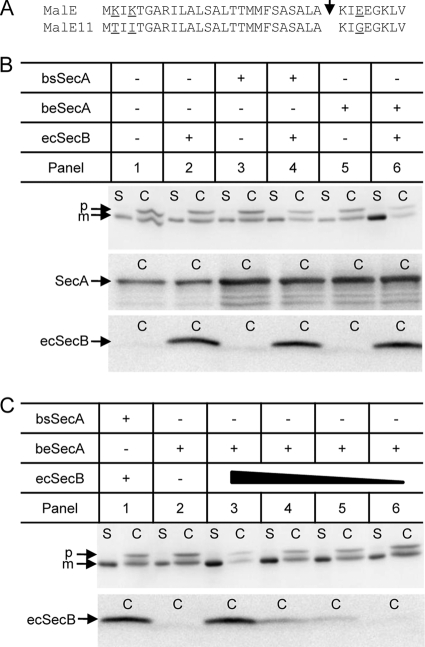
Fig 3.
Improvement of MalE11 secretion in B. subtilis by coexpression of ecSecB and beSecA. (A) Amino acid alterations present in the inefficiently secreted MalE11 protein. MalE, primary sequence of wild-type MalE corresponding to the signal peptide and the early mature region. MalE11, MalE mutant protein possessing mutations K2T and K4T in the signal peptide and mutation E29G in the early mature protein. The corresponding mutations result in a reduction of the positive net charge of the n-region of the signal peptide and in a more positively charged early mature protein domain, both of which are known to slow down the kinetics and overall efficiency of MalE membrane translocation in E. coli (28). The respective amino acid positions are underlined. The arrowhead indicates the signal peptidase cleavage site. (B) MalE11 secretion is enhanced in the presence of ecSecB and beSecA. Cellular (C) and supernatant (S) fractions of B. subtilis expressing the SecA and SecB proteins as indicated above the respective panels were subjected to SDS-PAGE and Western blotting using MalE antibodies (upper). In addition, the cellular fractions were immunoblotted using B. subtilis SecA antibodies (middle) or E. coli SecB antibodies (lower). p, MalE11 precursor protein; m, mature MalE11 protein. (C) Secretion stimulation of MalE11 in the presence of beSecA correlates to ecSecB availability. The amount of ecSecB, as symbolized by the solid triangle, in the cell was modulated by varying the concentration of the inducer xylose in the growth medium. The xylose concentrations used were 0.5% (panel 3), 0.1% (panel 4), 0.05% (panel 5), and 0% (panel 6), respectively. MalE11-derived protein products in the C and S fractions, as well as the amount of ecSecB in the C fractions of the respective cells, were analyzed by immunoblotting as described for panel B.