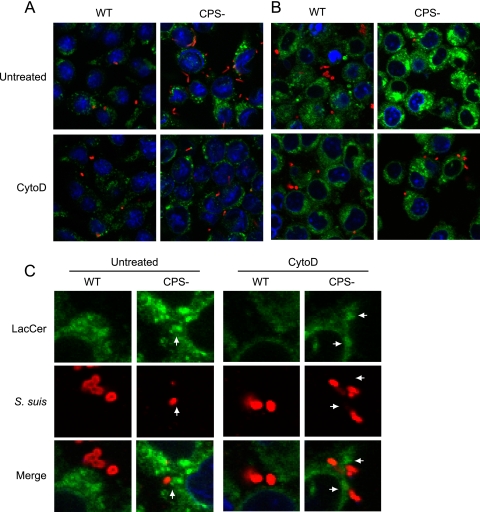
Fig 6.
S. suis CPS blocks pathogenic pattern recognition by LacCer in lipid microdomains of macrophages. J774 macrophages were either pretreated or not with CytoD and then infected with WT S. suis or its nonencapsulated mutant strain (CPS−) for 1 h. (A) Cells were stained with rat anti-LAMP1 antibody to show bacterial internalization (green) and with rabbit anti-S. suis antibodies (red) to stain bacteria. Compared to WT S. suis-infected macrophages, confocal analyses showed an increase of the intracellular CPS− mutant in untreated macrophages. CytoD blocked phagocytosis of S. suis by macrophages. (B) LacCer-BODIPY (green) was added to cells for 30 min, allowing integration of LacCer within the plasma membrane. In parallel, cells were either treated or not with CytoD. After 30 min, cells were washed to remove unincorporated LacCer, and macrophages were infected for 1 h with either S. suis WT strain or its CPS− mutant. Rabbit anti-S. suis antibodies (red) were used to stain bacteria. Untreated cells showed an increase in the intensity and size of LacCer-positive vesicles when macrophages were infected with the CPS− mutant. CytoD blocked LacCer label intensity by preventing internalization of S. suis by macrophages. (C) Closeup confocal analysis of the interaction of S. suis with LacCer at the plasma membrane of macrophages shown in panel B. Arrows indicate the phagocytic cup and close proximity of LacCer-positive membranes (green) with CPS− S. suis (red) adhered to the cell surface. WT S. suis shows no close proximity and less intensity of LacCer in the region directly adjacent to the bacteria. Inhibition of phagocytosis by CytoD reduced accumulation of LacCer at the phagocytic cup and prevented the increase of LacCer intensity in internal vacuoles.