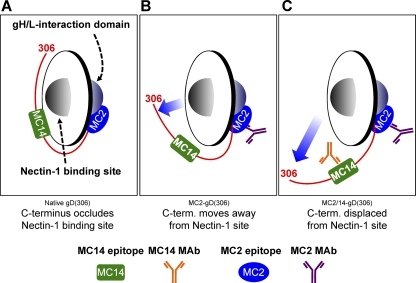
Fig 10.
Model depicting the receptor-binding and fusion activation domains of gD relative to the MC2 and MC14 epitopes. The Ig-like core of gD (residues 56 to 184) is represented as a disc. Each side of the disc represents a distinct protein interaction site. One side of gD interacts with cellular receptors (i.e., nectin-1), while the opposite side interacts with downstream elements of the HSV membrane fusion machinery (i.e., gH/gL). The C terminus (residues 240 to 306) is depicted as a red line extending from one side of gD to the other around the base of the Ig-like gD core. The MC2 and MC14 epitopes are represented by a blue oval and a green rectangle, respectively. (A) Native gD with the C terminus positioned across the nectin-1 binding site. In this conformation, receptor access is subjected to competition by the gD C-term. (B) The binding of either MC2 (shown) or MC14 to gD alters the gD conformation such that receptor binding is enhanced. The most likely explanation is that the C-term is displaced from its native conformation (movement is shown by the blue arrow). (C) When MC2 and MC14 are both bound to gD, the effect on gD structure is more profound. One manifestation of this structural alteration is an even greater increase in nectin-1 binding. We hypothesize that the neutralizing activity of MC2 is a result of the proximity of its epitope to the fusion activation site. Perhaps MC14 augments MC2-mediated neutralization by imposing additional conformational constraints on the fusion activation domain. Alternatively, MC14 may simply enhance MC2 binding or Ab:Ag complex stability.