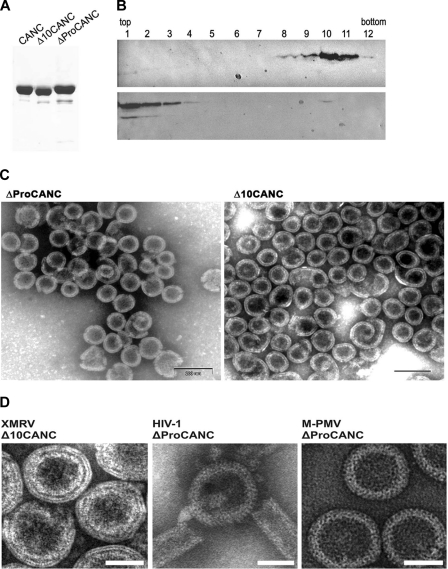
Fig 3.
(A) SDS-polyacrylamide analysis of purified XMRV proteins (Coomassie blue-stained gel). (B) Western blot analysis of in vitro-assembled XMRV particles in sucrose gradient fractions in the presence (upper blot) or in the absence (lower blot) of oligonucleotides. Δ10CANC protein (60 μg) was mixed with 6 μg of oligonucleotide 40-mer, dialyzed overnight against the assembly buffer, and centrifuged to equilibrium through a linear 20 to 65% sucrose gradient. The fractions were analyzed by Western blotting with rabbit anti-XMRV CANC antibodies. The sucrose densities in fractions 8 to 12 were as follows: 8, 1.19 g/ml; 9, 1.21 g/ml; 10, 1.22 g/ml; 11, 1.23 g/ml; 12, 1.25 g/ml. (C) TEM images of negatively stained in vitro-assembled material from XMRV ΔProCANC (left) and XMRV Δ10CANC (right). (D) TEM images of negatively stained VLPs assembled in vitro from XMRV Δ10CANC, HIV-1 ΔProCANC, and M-PMV ΔProCANC. Scale bars,e 50 nm.