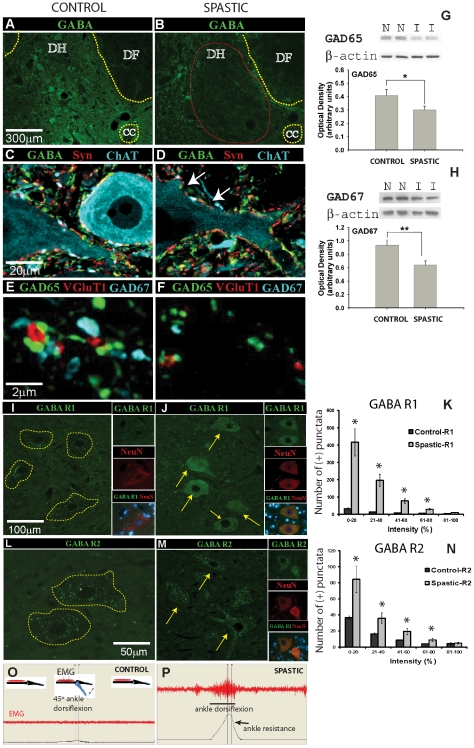
Figure 1. Loss of segmental inhibitory GABA-ergic interneurons and increased expression of GABA B R1+R2 receptor in α-motoneurons after transient spinal cord ischemia is associated with the development of chronic muscle spasticity.
(A, B) Transverse spinal cord sections taken from L2–L5 segments in control (A) or spinal ischemia-induced-spastic rat (B) at 24 h after intrathecal colchicine injection and stained for GABA. Note an apparent loss of GABA-ergic interneurons in the intermediate zone in spastic rat (B; red circle). (C–F) Loss of GABA-ergic interneurons corresponds with loss of GABA-IR and GAD65-IR boutons on membranes of persisting CHAT-IR α-motoneurons in animals with ischemic spasticity (white arrows). (G, H) Western blotting for GAD65 and GAD67 in lumbar spinal cord samples taken from control animals (n = 5–6) or animals with developed ischemic spasticity (n = 5–6), (*P = 0.017; **P = 0.045, unpaired t-test). (I–N) In comparison to control animals, an upregulation in GABA B R1+R2 receptors in lumbar α-motoneurons was identified in animals with spasticity (compare Fig. 1I to Fig. 1J and Fig. 1L to Fig. 1M ). Quantitative densitometric analysis showed significantly increased densities for both receptor subunits in spastic animals (*-P<0.05; Fig. 1K and Fig. 1N ). (O, P) Measurement of EMG activity in gastrocnemius muscle and corresponding ankle resistance during computer-controlled ankle rotation (45°/3 sec) in awake control sham-operated animals (O) and in animals with ischemic spasticity (P).