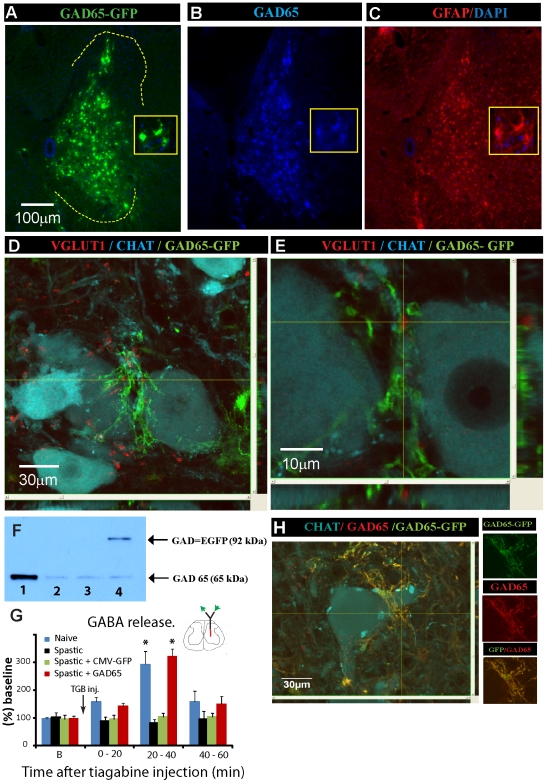
Figure 4. Spinal parenchymal injections of HIV1-CMV-GAD65-GFP lentivirus leads to increased GAD65 expression in infected astrocytes in rat and minipig and is associated with increased extracellular GABA release after tiagabine treatment in rats with ischemic spasticity.
(A–C) Immunofluorescence images taken from a transverse lumbar spinal cord section of a spastic rat at 3 weeks after spinal injection of HIV1-CMV-GAD65-GFP lentivirus. Sections were stained with GFP, GAD65 and GFAP antibody. (D, E) Confocal images demonstrating the localization of GAD65-GFP (green) expressing processes in HIV1-CMV-GAD65-GFP-infected cells surrounding VGLUT1 (red)-IR primary afferent terminals in the vicinity of persisting CHAT (blue)-IR α-motoneurons. (F) Western blot analysis for GAD65 in spinal cord homogenate taken from lumbar spinal parenchyma of naive-control (column 1) spastic non-treated (columns 2 and 3) and spastic HIV1-CMV-GAD65-GFP-injected animal (column 4). (G) Extracellular GABA concentration measured by intraparenchymal microdialysis in lumbar gray matter in naive (n = 6), ischemic-spastic (n = 6), ischemic-spastic-HIV1-CMV-GFP (n = 6) and ischemic-spastic-HIV1-CMV-GAD65-GFP (n = 6) lentivirus-injected animals before and after systemic tiagabine (40 mg/kg) injection. A significant increase in extracellular GABA concentration was measured at 20–40 min after tiagabine administration in naive animals and ischemic-spastic animals previously injected spinally with HIV1-CMV-GAD65-GFP lentivirus (P<0.05; paired t test). (H) Confocal images of transverse spinal cord section taken from a minipig lumbar spinal cord at 2 months after spinal HIV1-CMV-GAD65-GFP injections and stained with GFP, GAD65 and CHAT antibody.