Figure 1. Human Rad9 structural and functional domains.
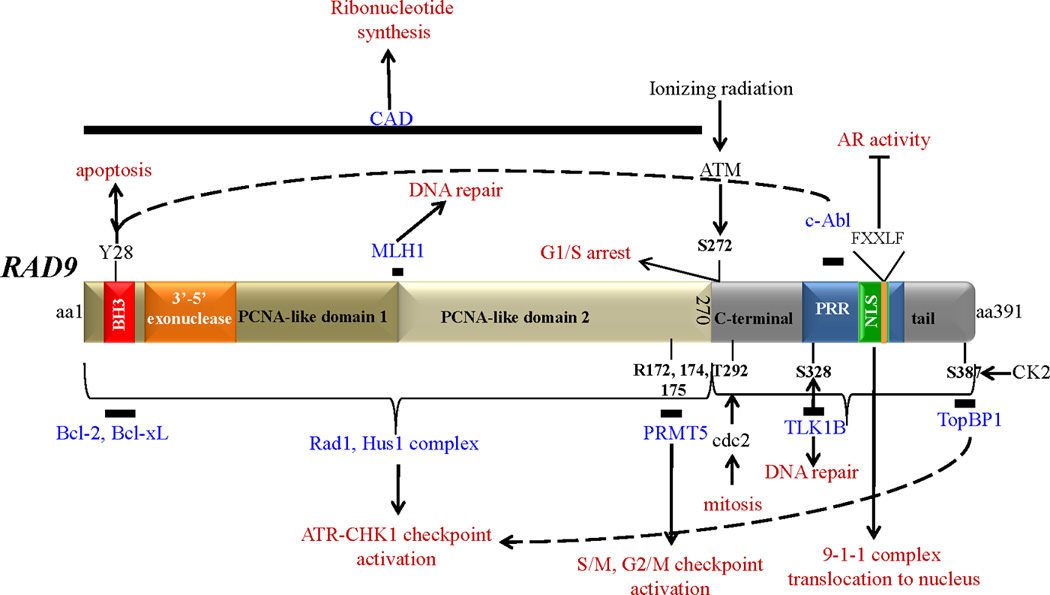
Rad9 is a 391 aa protein, roughly divided into two parts; the N-terminal aa1-270 contains two PCNA-like domains, primarily involved in assembling the 9-1-1 complex, and the C-terminal tail domain aa271-391 that does not participate in the 9-1-1 complex, but binds a number of proteins crucial for its function, is highly phosphorylated, and contains a proline-rich domain (PRR) and a nuclear localization signal (NLS). Solid bars denote the position of Rad9 that interacts with the depicted proteins (shown in blue). Proteins that Rad9 interacts with, but their exact binding position is not known, are not shown. FXXLF is the Rad9 sequence that binds to AR. The functional outcome of Rad9-protein association is shown in red. Phosphorylation sites: Y28, S272, T292, S328, S387.
Methylation sites: R172, R174, R175. Abbreviations are as follows: BH3: bcl-2 homology motif 3; PCNA: proliferating cell nuclear antigen; PRR: proline-rich domain; NLS: nuclear localization signal; PRMT5: protein methyltransferase 5; TLK-1B: tousled-like kinase 1B; TopBP1: topoisomerase IIb binding protein; MLH1: mutL homolog 1; CAD: carbamoyl phosphate synthetase/aspartate transcarbamoylase/dihydroorotase; AR: androgen receptor; CK2: casein kinase 2; ATM: Ataxia telangiectasia mutated; ATR: ATM and Rad3-related; CHK1: checkpoint kinase 1.
