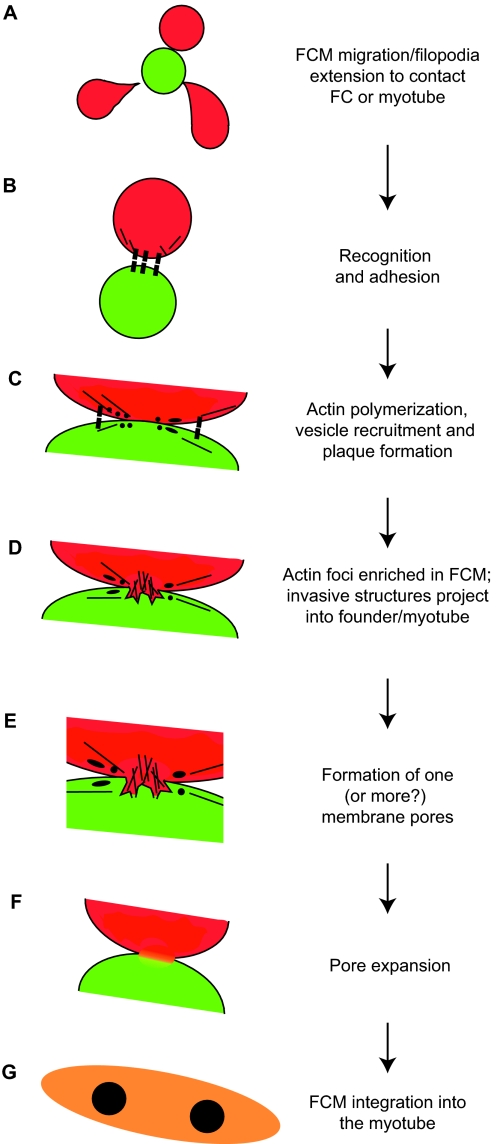
Fig. 4.
Hypothetical model of myoblast fusion in Drosophila embryos. (A) A fusion-competent myoblast (FCM; red) migrates or extends filopodia to contact a founder cell (FC; green) or, in subsequent rounds of fusion, a syncytial myotube (orange). (B) Cell-surface adhesion molecules (black boxes) mediate recognition and adhesion between cells. (C) Following cell-cell contact, and prior to fusion, electron-dense vesicles (black circles) are recruited to points of contact, possibly through vesicle trafficking mechanisms from the Golgi (not shown). This process may involve actin filaments (black lines). Such vesicles facilitate the fusion process, possibly by delivering fusion-associated components, such as lipids, fusogens or proteases, via targeted exocytosis near or at the sites of fusion. Vesicles may give rise to membrane plaques (black ellipses), which could reflect accumulation of adhesion proteins or other fusion machinery. (D) Actin accumulates in the FCM, forming a large F-actin-based protrusion that pushes into the founder cell. A thin sheath of actin is present in the founder cell (not shown). (E) One, or more, fusion pores form to allow mixing of cytoplasmic contents. (F) Expansion of the fusion pore(s) and elimination of membrane separating the cells. (G) The FCM is absorbed into the myotube, and the resulting syncytium continues additional rounds of fusion as needed.