Table 1.
Structures, XI(50) values, calculated binding affinity (−logKd), polar score that represents the contribution of hydrogen bonding to the calculated binding affinity, and the hydrophobic score that represents the contribution of the hydrophobic interactions to the calculated binding affinity for the 2-oxoamide inhibitors are shown.
| Code | Molecular Structure | XI(50) | −logKd | Polar | Hydrophobic |
|---|---|---|---|---|---|
| AX074 |
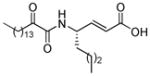
|
0.003a | 11.94 | 4.87 | 7.07 |
| AX109 |
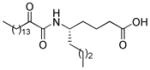
|
0.005b | 10.11 | 2.98 | 7.13 |
| AX007 |
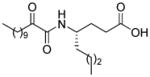
|
0.009c | 9.26 | 3.49 | 5.77 |
| AX073 |
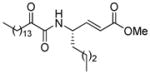
|
0.018a | 8.24 | 3.17 | 5.07 |
| AX063 |
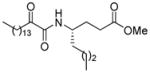
|
0.019b | 8.20 | 2.57 | 5.63 |
| AX013 |
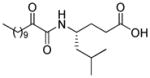
|
0.021d | 7.84 | 3.17 | 4.67 |
| GK165 |
|
0.023e | 7.53 | 3.79 | 3.74 |
| AX006 |
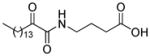
|
0.024f | 7.21 | 4.08 | 3.13 |
| AX021 |
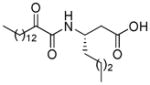
|
0.027b | 7.10 | 2.77 | 4.33 |
| AX016 |
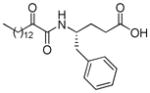
|
0.035d | 7.19 | 2.76 | 4.43 |
| AX008 |
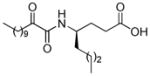
|
0.068c | 5.25 | 2.90 | 2.35 |
