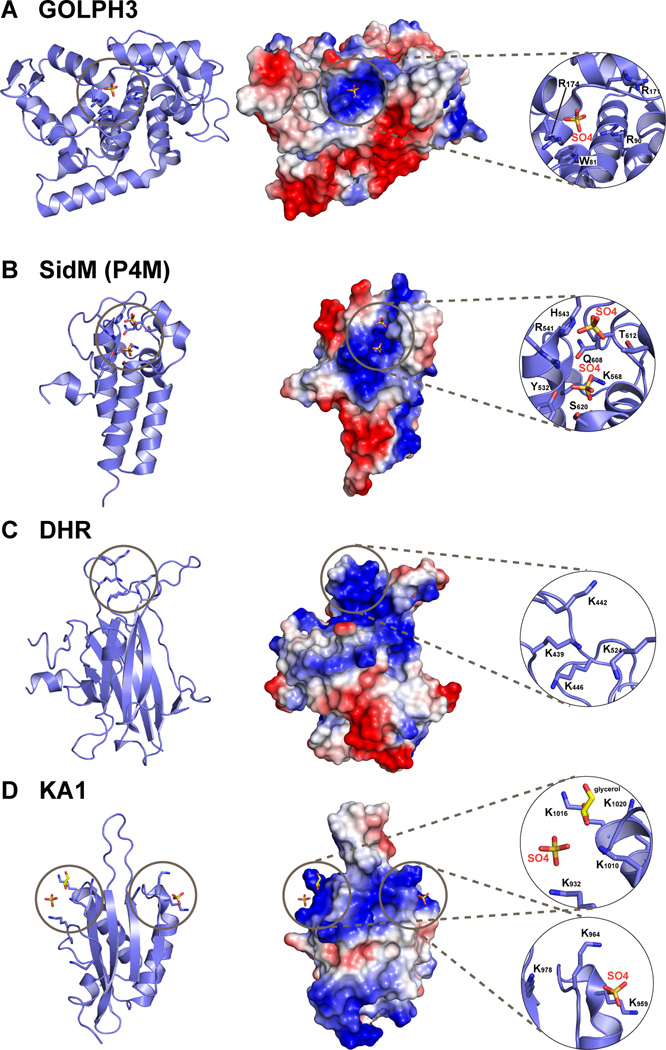
Figure 3. Recent additions to the list of phospholipid binding domains.
Structures of newly reported phospholipid-binding domains are shown in cartoon representation (left); in surface representation (middle) colored according to electrostatic potential (blue is positive, red is negative); and with a close-up view of the proposed lipid or anion binding sites (right). A. The helical GOLPH3 protein (Wood et al., 2009), from PDB entry 3KN1. A sulfate ion lies in the basic patch implicated in PtdIns4P binding. B. The P4M PtdIns4P-binding domain of the Legionella pneumophila SidM protein, from PDB entry 3N6O, which also includes an adjacent GEF domain that is not shown (Schoebel et al., 2010). Two bound sulfate ions are marked. C. The uncomplexed Dock Homology Region-1 (DHR-1) domain (Premkumar et al., 2010) from PDB entry 3L4C. D. The KA1 domain from the S. cerevisiae Kcc4p kinase (Moravcevic et al., 2010) from PDB entry 3OST, which has two primary regions of positive charge on its surface that contribute to non-specific association with negatively-charged membrane surfaces, each with a bound sulfate ion.