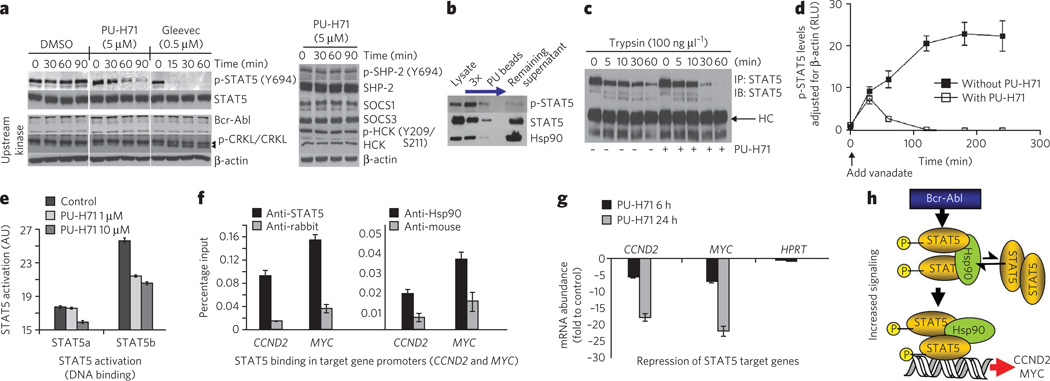
Figure 5. Hsp90 facilitates an enhanced STAT5 activity in CML.
(a) Representative western blot of K562 cells treated for the indicated times with PU-H71 (5 µM), Gleevec (0.5 µM) or DMSO (vehicle). (b) Representative western blot of sequential chemical precipitations conducted in K562 cells with PU and control beads, as indicated by the blue arrow. (c) Representative western blot of STAT5 immunocomplexes from cells pretreated with vehicle or PU-H71, and then treated for the indicated times with trypsin. (d) p-STAT5 concentrations in K562 cells treated for the indicated times with vanadate (1 mM) in the presence and absence of PU-H71 (5 µM). Data are presented as mean ± s.d. (n = 3). (e) The DNA-binding capacity of STAT5 in K562 cells treated for 24 h with the indicated concentrations of PU-H71. (f) Quantitative ChIP performed with STAT5 or Hsp90 antibodies versus an IgG control for two known STAT5 target genes. A primer that amplifies an intergenic region was used as negative control. Results are expressed as a percentage of the input for the specific antibody (STAT5 or Hsp90) over the respective IgG control. (g) The transcript abundance of CCND2 and MYC in K562 cells exposed to µM of PU-H71. Results are expressed as fold change compared to baseline (time 0 h) and were normalized to RPL13A. HPRT was used as negative control. Data are presented as means ± s.e.m. (h) Proposed mechanism for Hsp90-facilitated increased STAT5 signaling in CML. Hsp90 binds to and influences the conformation of STAT5 and maintains STAT5 in an active conformation directly within STAT5-containing transcriptional complexes.