Figure 1.
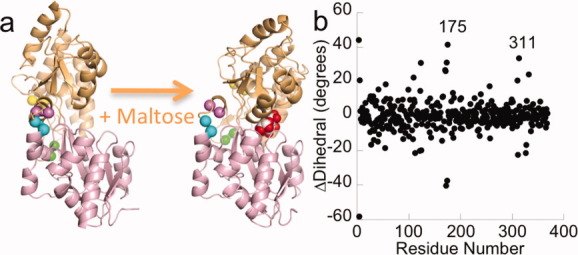
Conformational changes in MBP upon maltose binding. Cartoon representation (a). The residues between which cpGFP was inserted are identified with colored spheres at the Cα positions. Yellow: 165–166, green: 175–176, cyan: 311–312, and violet: 317–318. Figure was made with PyMOL.41 Analysis of backbone structural changes (b). The Cα dihedral is calculated from the four atoms: Cαi+2, Cαi+1, Cαi, Cαi−1. ΔDihedral is calculated as the difference in dihedrals between the closed (1ANF) and open (1OMP) states of MBP and corrected to fall within a range of −180° to 180°. The regions near residues 175 and 311 are labeled. There is a crystallographic artifact at the N-terminus resulting in the appearance of significant structural changes.
