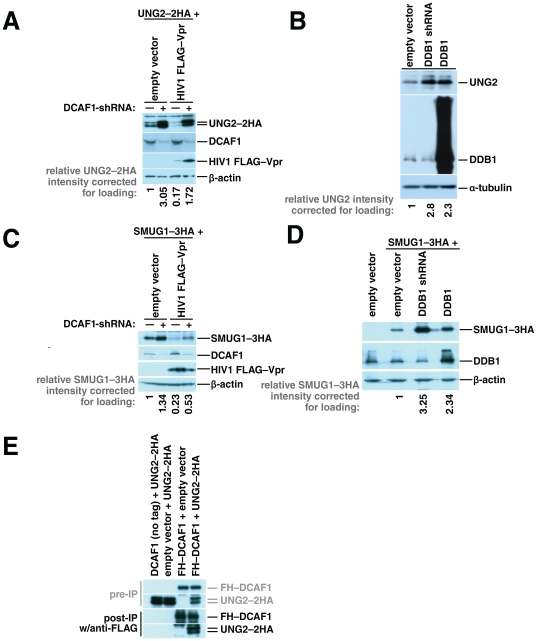
Figure 1. CRL4DCAF1 is involved in constitutive turnover of UNG2 and SMUG1.
Cultures of 293T HEK cells were transfected with 1 µg of UNG2–2HA expression vector, together with 2 µg of empty vector or 2 µg of an expression vector for DCAF1-directed shRNA and additional empty vector (1 µg) or HIV1 FLAG–Vpr expression vector (1 µg). 48 hours after transfection cell lysates were harvested and tested for expression of UNG2–2HA, HIV1 FLAG–Vpr, endogenous DCAF1 and β-actin by immunoblotting (A). 293T HEK cells were transfected with empty vector (4 µg), or expression vector for DDB1 (4 µg) or DDB1-directed shRNA (4 µg) as indicated. Fourty-eight hours after transfection cell lysates were prepared and tested for expression of endogenous UNG2, DDB1, and α-tubulin by immunoblotting (B). Cultures of 293T HEK cells were transfected with SMUG1-3HA expression vector (1 µg), together with empty vector (2 µg) or an expression vector for DCAF1-directed shRNA (2 µg) and additional empty vector (1 µg) or HIV1 FLAG–Vpr expression vector (1 µg). 48 hours after transfection cell lysates were harvested and tested for expression of SMUG1–3HA, HIV1 FLAG–Vpr, DCAF1 and β-actin by immunoblotting (C). 293T HEK cells were transfected with empty vector (1 µg) or SMUG1–3HA expression vector (1 µg) together with additional empty vector (3 µg) expression vectors for DDB1 (3 µg) or DDB1-directed shRNA (3 µg) as indicated. 48 hours after transfection cell lysates were prepared and tested for expression of SMUG1-3HA, DDB1, and β-actin by immunoblotting (D). 293T HEK cells were transfected with UNG2–2HA expression vector, together with empty vector or an expression vector for untagged DCAF1 or transfected with FLAG–HA–DCAF1 expression vector, together with empty vector or an expression vector for UNG2–2HA. After 48 hours, the cells were lysed and the cleared lysates were incubated with anti-FLAG agarose beads. The bound proteins were eluted with FLAG peptide. The eluted proteins and pre-immunoprecipitation samples were characterized by immunoblotting with HA-specific antibody (E).