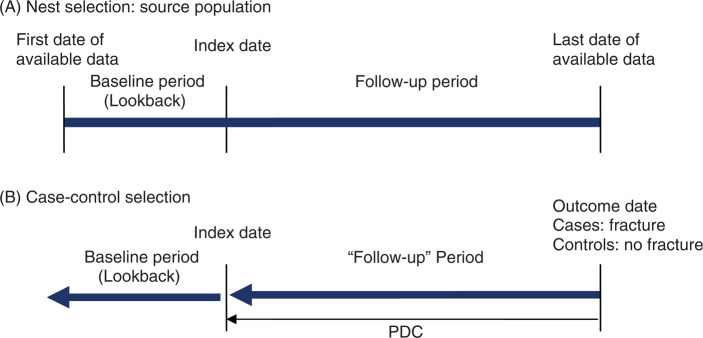
Figure 2.
Depiction of a nested case—control study design to measure compliance and impact on fracture risk. Index date is identified by the prescription date that defines cohort entry. (A) The source population (‘nest’) is selected based upon study inclusion and exclusion criteria to identify osteoporosis treatment users. A baseline period prior to drug index date may be used to identify cohort eligibility. (B) Cases are selected as individuals within the nest with the outcome of interest (fracture) and controls as those individuals matched to cases with no fracture. Both cases and controls are followed back until the date of the first prescription (index date), with compliance measured over this period of time (‘follow-up’ period). Covariates are determined from the baseline (lookback) period prior to first treatment and/or during follow up. PDC, proportion of days covered (measure of compliance) = total days of drug supplied in the observation period divided by the total days in the observation period and capped at 100%.