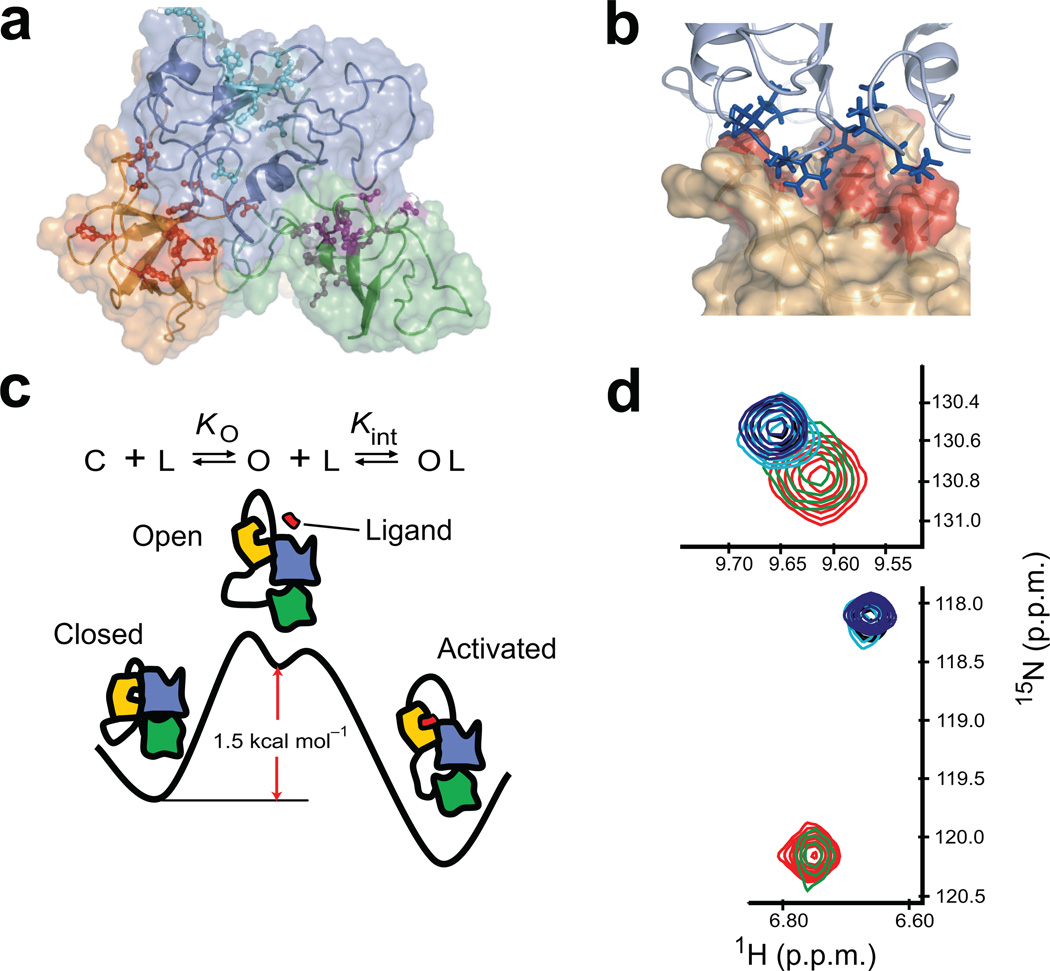
Figure 1. Scheme for the autoinhibition and activation of Crk-II.
(a) Solution structure of Crk-II (SH2; blue, nSH3; yellow, cSH3; green) (pdb id; 2EYZ19). Active site residues of each domain are shown in stick-and-ball format. (b) Expanded view of the interface between the active site of the nSH3 and SH2 domain in Crk-II. Active site residues are shown in red on the surface representation of the nSH3 domain. Interface residues of SH2 domain are shown in stick format. (c) Schematic representation of a three-state equilibrium of ligand binding to the nSH3 domain in Crk-II (SH2; blue, nSH3; yellow, cSH3; green, ligand; red). KO is the equilibrium constant between the closed and open state, and Kint is the intrinsic binding affinity of the ligand to the nSH3 domain in the open state. C and O represent the closed and open state of Crk-II, respectively. L represents the ligand that binds to the nSH3 domain. (d) Change in the chemical shift of W169 (upper panel) and A172 (lower panel) as a function of C3G-ligand concentration. The ratios of protein and ligand are 1:0 (red), 1:0.22 (green), 1:0.63 (cyan), 1:0.79 (black) and 1:1.19 (blue).