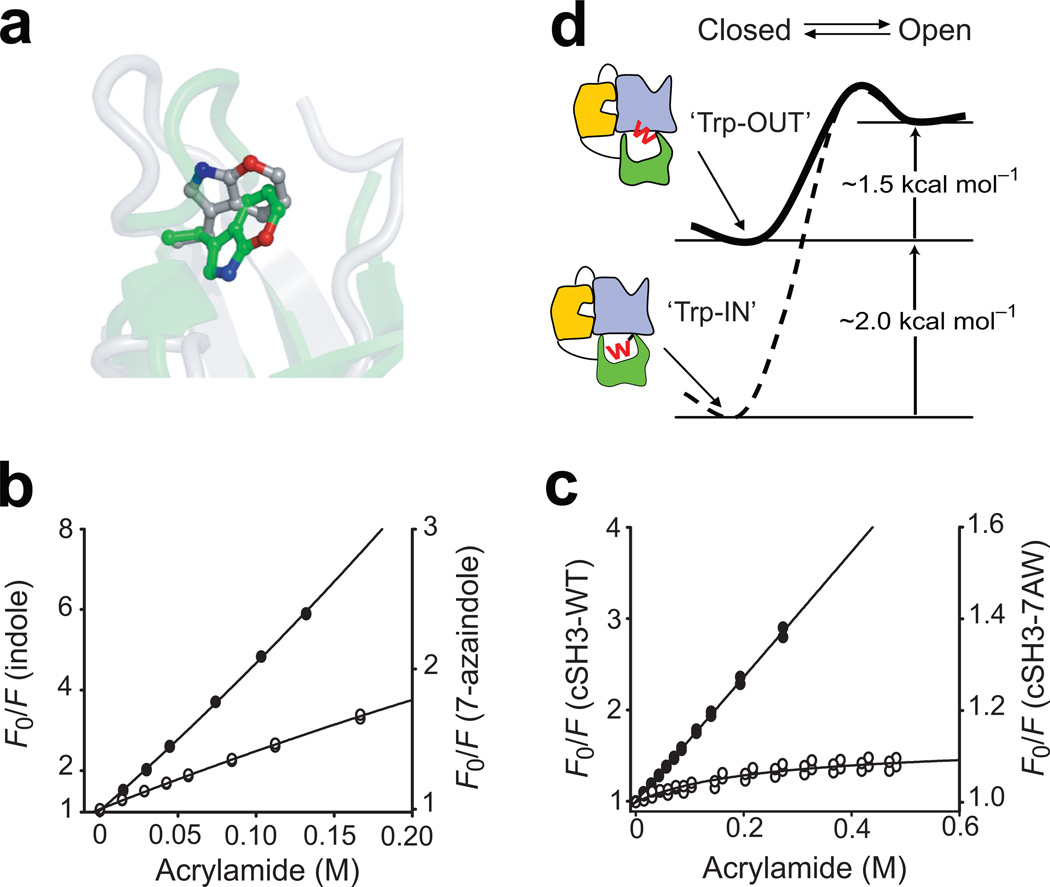
Figure 4. Conformation of Trp275 modulates the stability of the cSH3 domain.
(a) Comparison of the position of the indole ring of Trp275 in the structures of isolated cSH3 domain (green, pdb id: 2GGR32) and full-length Crk-II (silver, pdb id: 2EYZ19). The 7-position of the Trp residue is highlighted in red. Steady-state fluorescence quenching experiments for (b) free indole (closed circles) and 7-azaindole (open circles) and (c) isolated cSH3-WT (closed circles) and isolated cSH3-7AW (open circles). The solid lines represent the best fit model using the Stern-Volmer relationship (Methods). (d) Schematic representation of the effects of the conformational change of Trp275 on the equilibrium between the open and closed states of Crk-II (W represents Trp275). Reduced stability of the cSH3 upon interdomain interactions reduces the activation barrier between the open and closed states of Crk-II.