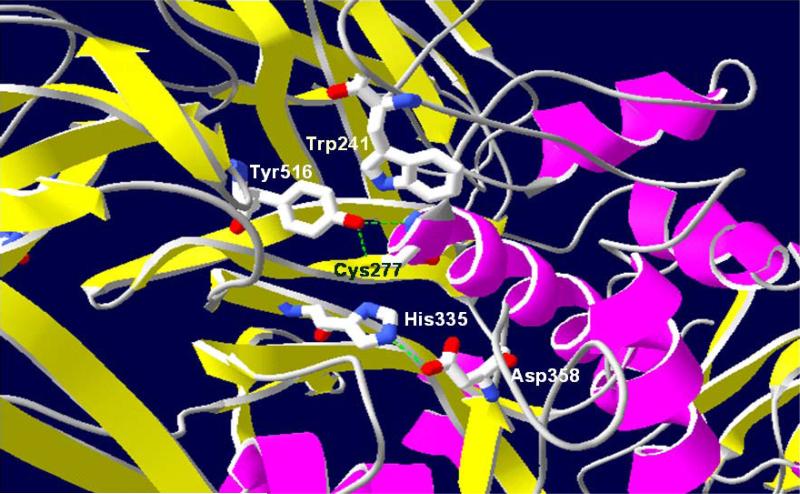
Figure 3. Transamidase active site of TG2.
The catalytic site of transamidating activity is composed of the catalytic triad: Cys277, His335 and Asp358. A conserved tryptophan residue, Trp241 is also critical for the transamidating activity. A hydrogen bond forms between Cys277 and Tyr516 in the closed conformation of TG2, which is believed to further stabilize the closed conformation and keep the enzyme inactive.