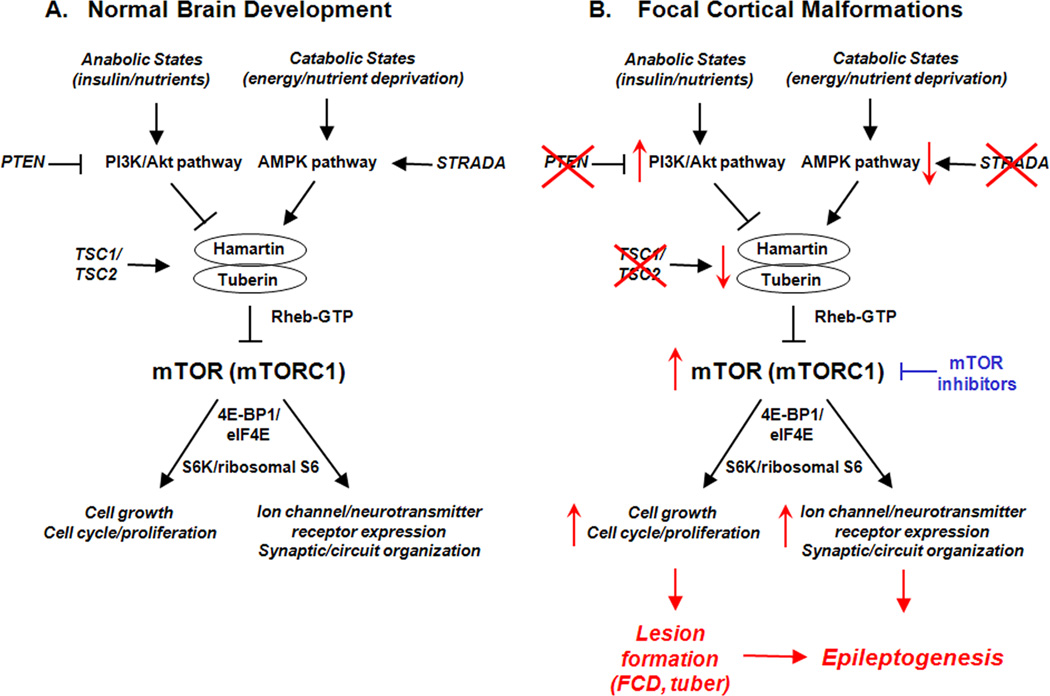
Figure 1.
Putative role of the mTOR pathway in pathogenesis and epileptogenesis of focal cortical malformations. A. During normal brain development, the mTOR pathway, particularly mTORC1, regulates a number of important physiological functions, such as cell growth, proliferation, ion channel expression, and synaptic and circuit plasticity, primarily via activation of protein synthesis mechanisms, such as through S6K/ribosomal S6 protein and eukaryotic initiation factor 4E binding protein-1 (4E-BP1)/eukaryotic initiation factor eIF4E pathways. In turn, in response to different physiological conditions and stimuli, mTORC1 may be activated or inhibited by various upstream signaling pathways, such as the insulin/phosphoinositide 3-kinase (PI3K)/Akt pathway or the adenosine monophosphate-activated protein kinase (AMPK) pathway. B. In focal malformations of cortical development, the mTOR pathway may become hyperactivated, caused in some cases by known mutations in upstream regulators of mTOR (e.g. TSC1 or TSC2 genes in the disease TSC directly affecting hamartin or tuberin expression; STRADA gene in PMSE syndrome, leading to decreased AMPK pathway activation; PTEN inactivation in an animal model of FCD, leading to increased PI3K/Akt pathway activity) or in other cases by unknown mechanisms (e.g. most cases of isolated FCD in people). Regardless of the initial upstream trigger, disinhibition or hyperactivation of the mTOR pathway can lead to abnormally increased cell growth and proliferation, which may account for the focal lesion formation of FCD and tubers. The gross structural lesions themselves, as well as non-structural molecular and cellular changes in ion channel expression and synaptic organization triggered by altered mTOR-mediated protein synthesis, may promote epileptogenesis in these disorders. Finally, mTOR inhibitors may represent a rational therapy for FCD and TSC by reversing mTORC1 hyperactivation and the downstream mechanisms of epileptogenesis.