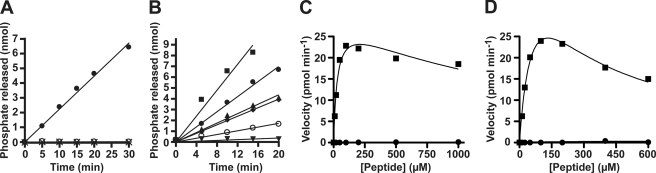
FIGURE 1.
Budding yeast Cdc14 is highly selective for Ser(P) at Cdk phosphorylation sites in phosphopeptide substrates. A, time-dependent dephosphorylation of phosphopeptide substrates Acm1pS3 (●), Acm1pT161 (○), and Cdh1pT157 (X) by budding yeast Cdc14. The concentration of all substrates was 300 μm. B, same as A showing Ser(P)-containing peptide substrates Acm1pS3 (●), Acm1pS31 (■), Acm1pS48 (▴), Cdh1pS42 (⧫), Cdh1pS169 (▾), and Cdh1pS239 (○). All substrates were 100 μm. Peptide sequences are shown in Table 1 and supplemental Table S1. C and D, the rate of dephosphorylation of peptides Acm1pS31 (■) and Acm1pT31 (●) (C) and of peptides Cdc6pS7 (■) and Cdc6pT7 (●) (D) was measured as a function of peptide concentration. Data are the average of three independent experiments, and using non-linear regression, data were fit with a form of the Michaelis-Menten equation containing a substrate inhibition term. The amino acid sequence and kinetic parameters for peptides in C and D are given in Table 1 or supplemental Table S2.