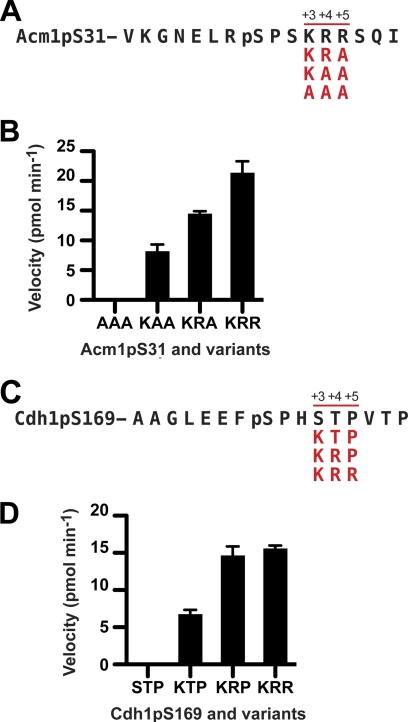
FIGURE 3.
Activity of Cdc14 phosphatases is dependent on basic residues C-terminal to Ser(P) at Cdk sites. A and C, the complete sequences of the wild-type Acm1pS31 (A) and Cdh1pS169 (C) peptides are shown in black on the first line with the residues of the +3 to +5 region (relative to Ser(P) (pS)) in each variant shown below in red. B and D, rates of dephosphorylation of Acm1pS31 and its variants (B) and of Cdh1pS169 and its variants (D) by budding yeast Cdc14 were compared at a single substrate concentration of 300 μm. The amino acid sequence of the +3 to +5 region in each peptide is shown below the x axis. Data represent the mean of three independent experiments with standard errors.