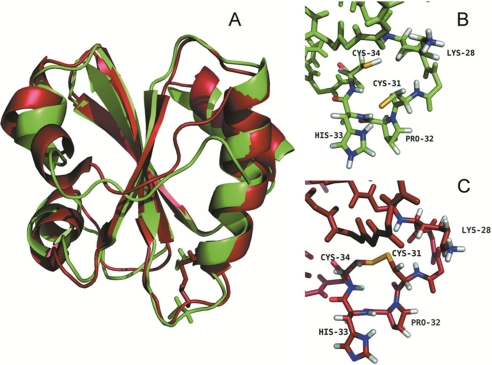
FIGURE 4.
Three-dimensional structure of Dtrx in both redox states. A, overlay of the three-dimensional solution structures of the reduced (green) and oxidized (red) forms of Dtrx calculated with CYANA (21). The NMR sample contained 1 mm protein concentration (90% H2O, 10% D2O) in 100 mm NaCl, 50 mm phosphate buffer, pH 5.7, at 290 K. For reduced Dtrx, the intramolecular disulfide bond was reduced by adding DTT to a final concentration of 10 mm, under argon atmosphere. The Dtrx typical thioredoxin fold is represented in cartoon, and the side chains of the cysteine residues are shown in sticks. B, local conformations of the active site in the reduced form of Dtrx. C, local conformations of the active site in the oxidized form of Dtrx. The active site residues Cys31, Pro32, His33, and Cys34 are shown and labeled. Sulfur atoms are shown in yellow, hydrogen atoms are in white, and nitrogen and oxygen atoms are in blue and red, respectively. The figures were generated using the PyMOL Molecular Graphics System, Version 1.2r3pre, Schrödinger, LLC.