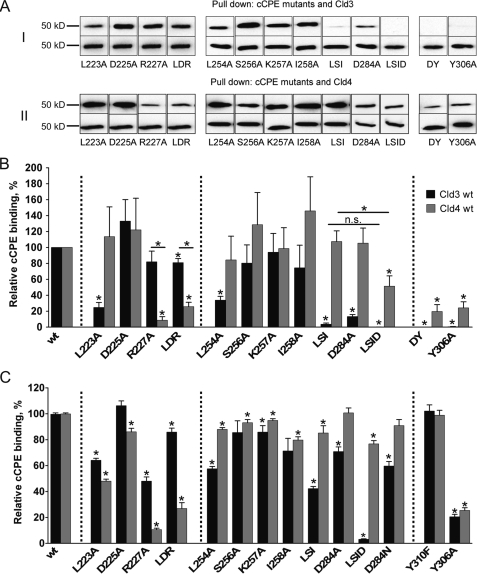
FIGURE 2.
cCPE-binding to full-length Cld3 and Cld4 is affected differently by amino acid substitutions in cCPE. A, lysates of HEK cells transfected with Cld3WT or Cld4WT used for pulldown assays with GST-cCPE constructs. Bound fractions were analyzed by SDS-PAGE and Western blotting. Top rows of I and II show bands of Cld3 or Cld4 bound to particular GST-cCPE mutants, and bands in bottom rows show Cld3 or Cld4 bound to corresponding GST-cCPEWT (used as internal standard). Substitutions in upper (left panel) or lower (middle panel) rim of cCPE binding pocket and D284A/Y306A or Y306A (right panel) are shown. LDR, L223A/D225A/R227A; LSID, L254A/S256A/I258A/D284A; DY, D284A/Y306A. B and C, quantification of pulldown (B) and cellular binding assay (C). Results reflect mean ± S.E. (error bars); n ≥ 4. *, p < 0.05 to GST-cCPEWT. Dotted lines separate different groups of substitutions. For L254A/S256A/I258A/D284A and Cld3 in C, n = 2; additional data were quantified by Western blotting relative cCPE binding = 1.0% ± 0.9%, n = 8.