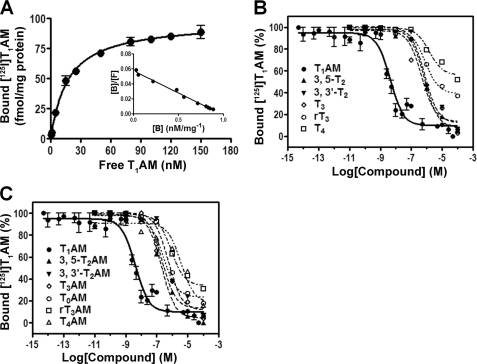
FIGURE 6.
Equilibrium binding of [125I]T1AM to apoB-100-containing lipoprotein particles. An equilibrium dissociation constant (KD) of 17 nm was determined from the fit (A). Scatchard analysis reveals a single and saturable binding site with a 1:1 stoichiometry between ligand and protein (inset of A). Competition of [125I]T1AM binding to apoB-100-containing lipoprotein with unlabeled thyronines (B) and thyronamines (C) is shown. For competition binding assays, apoB-100-containing lipoprotein was incubated with tracer quantity of [125I]T1AM in the presence or absence of excess T1AM and the indicated concentrations of competitor ligand for 24 h at 4 °C. Plotted data are the mean ± S.D. of three experiments.