FIGURE 10.
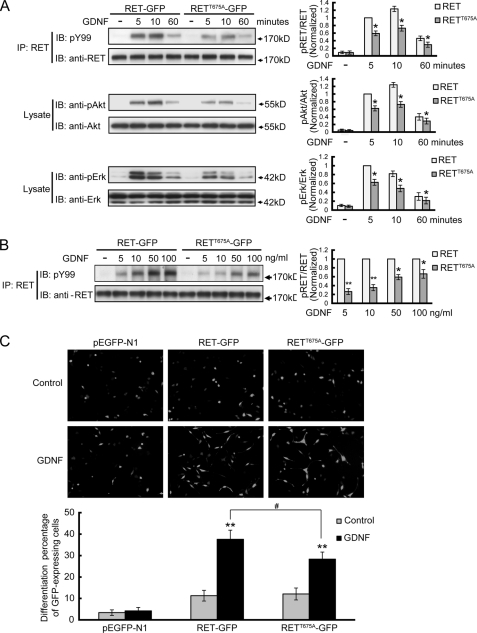
RET Thr675 residue regulates ligand-induced RET signaling and cell differentiation. A, analysis of the signal transduction mediated by RETT675A receptor in response to GDNF stimulation. PC12 cells were transfected with GFP fused RET or RETT675A and stimulated with 50 ng/ml GDNF for 5, 10 or 60 min. Immunoprecipitates or cell lysates were analyzed with the indicated antibodies. The levels of phospho-RET, phospho-Akt, and phospho-Erk1/2 were represented as the ratios of phosphoprotein over total protein. The histogram shows the phosphorylation levels of RET, Akt or Erk1/2 from three independent experiments normalized to their respective 5 min RET group. The results are represented as mean ± S.E. from three independent experiments (*, p < 0.05 versus their respective RET group; Student's t test). B, analysis of RET and RETT675A activation in response to different GDNF concentrations. PC12 cells were transfected with GFP-fused RET or RETT675A and stimulated by different GDNF concentrations. The levels of phospho-RET were represented as the ratios of phospho-RET versus total RET. At each GDNF concentration, phosphorylation levels of RETT675A were normalized to that of RET. The results are represented as mean ± S.E. from three independent experiments (*, p < 0.05, **, p < 0.01 versus their respective RET group; Student's t test). C, GDNF induced differentiation of PC12 cells expressing GFP fused RET or RETT675A. PC12 cells transfected with the indicated constructs were stimulated with 50 ng/ml GDNF for 3 days. Cells displaying neurite extension longer than twice the cell diameter were counted as differentiated cells. The results were normalized to the total number of transfected (GFP-positive) PC12 cells in each field. All of the GDNF stimulations were supplemented with 300 ng/ml soluble GFRα1. The results are represented as mean ± S.E. from three independent experiments (**, p < 0.01 versus their respective control group; #, p < 0.05 versus GDNF-treated RET group; one-way ANOVA).