FIGURE 9.
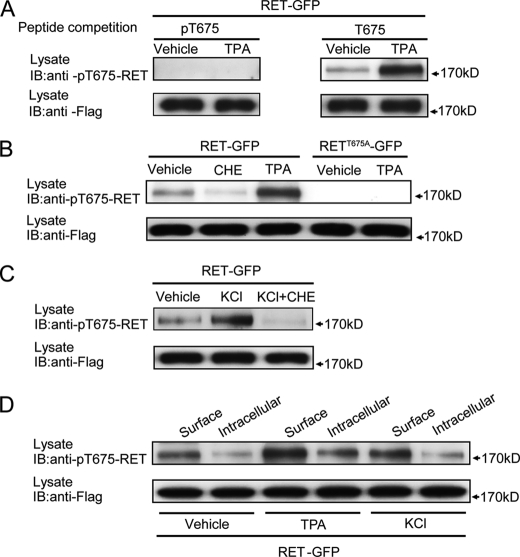
RET Thr675 residue is phosphorylated by PKC and depolarization. A, specificity of the rabbit anti-pT675-RET antibody was analyzed by a peptide competition experiment. B, RET Thr675 residue was phosphorylated by PKC in transfected PC12 cells. PC12 cells expressing RET-GFP were incubated with CHE for 2 h or TPA for 30 min, and then RET phosphorylation was detected by rabbit anti-pT675-RET antibody. Phosphorylation of RETT675A-GFP after TPA treatment was measured to confirm the binding specificity of our rabbit anti-pT675-RET antibody. C, phosphorylation of RET Thr675 residue upon depolarization in transfected PC12 cells was measured. PC12 cells expressing RET-GFP were treated with 50 mm KCl for 30 min in the absence or presence of CHE pre-treatment. Phospho-Thr675 levels were analyzed by Western blot. D, cell surface RET was preferentially phosphorylated on Thr675 compared with cytoplasmic RET. After incubation with TPA or KCl for 30 min, surface and cytoplasmic RET proteins in transfected PC12 cells were respectively collected. Thr675 phosphorylation levels were examined in surface or cytoplasmic RET fraction from vehicle, TPA, or KCl groups.