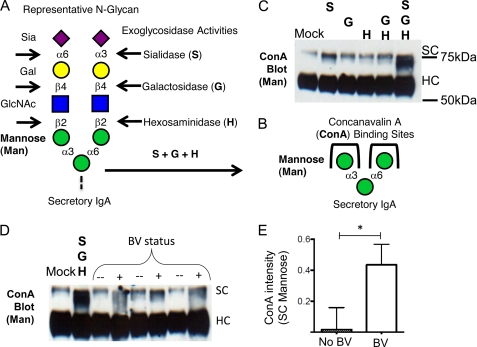
FIGURE 4.
Deglycosylation of SIgA reveals underlying sugars. A and B, a common trisaccharide motif on N-glycans of SC (sialic acid (Sia)–galactose (Gal)–N-acetyl glucosamine (GlcNAc)) can be digested by successive exoglycosidase activities, revealing otherwise cryptic α-linked mannose (Man) residues, which are recognized by the lectin ConA. C, ConA shows little binding to secretory component until Sia, Gal, and N-acetyl hexose have been removed by combined treatment with sialidase, galactosidase, and hexosaminidase. C, ConA reactivity is revealed upon treatment with commercial exoglycosidase enzymes. D, representative ConA blot following SIgA treatment with BV specimens and normal controls (n = 3 each). E, quantitation of ConA reactivity with secretory component (gel in four dimensions) revealed statistically significant differences between BV samples and normal controls. Gel bands were integrated using ImageJ with rectangles of equal size extending from the top of mock treated secretory component to just above heavy chain without background subtraction. The data were normalized relative to mock treated SC (set at 1). The data shown are representative of three experiments. Statistical significance was examined by unpaired t test.