FIGURE 7.
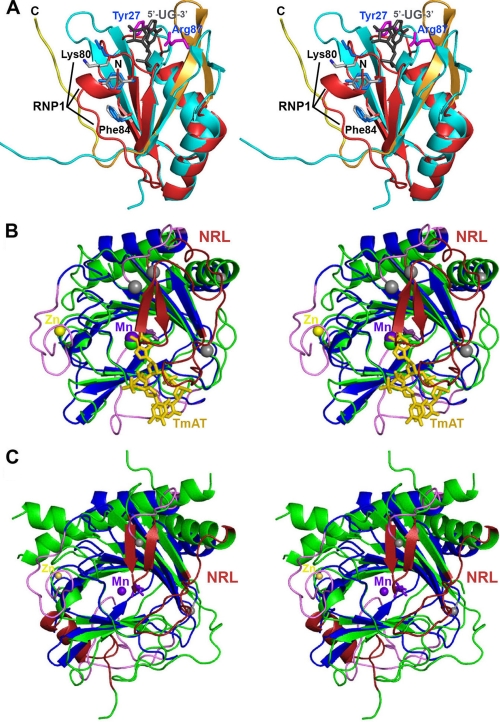
Structural alignments of human ABH8 domains. A, structural alignment (51) of the RRM domains from ABH8 and S. cerevisiae Rna15 (56). The backbone of the ABH8 RRM domain is colored red and orange as in Fig. 6A, and the residues in its RNP1 motif are highlighted and shown in gray in stick representation (with black labels except for Tyr82, which is unlabeled). The N terminus of the ABH8 domain is indicated by the black label “N.” The backbone of Rna15 is colored cyan; its crystallographically observed side chains interacting with RNA (magenta with blue labels), the consensus residues in its RNP1 motif (light blue), and its bound RNA ligand (gray) are all shown in stick representation. Note that this RNA ligand binds to Rna15 on the ridge of the RRM domain proximal to the AlkB domain in the structure of ABH8. The residues in the RNP1 motif are solvent-exposed in Rna15 but partially buried by the N-terminal α-helical segment in ABH8. B, structural alignment of the ABH8 AlkB domain with E. coli AlkB (35). ABH8 is colored as in Fig. 6A with gray spheres added to highlight the termini of its disordered backbone segments. Its bound Mn(II) and Zn(II) cations are shown, respectively, as purple and yellow spheres, whereas its bound 2OG co-substrate is shown in purple in stick representation (adjacent to the Mn(II) cofactor). The core of E. coli AlkB is colored green, its nucleotide-recognition lid (NRL) is colored red, and its bound DNA substrate (TmAT) is colored orange. C, structural alignment of the AlkB domains from ABH8 and human FTO with the latter colored like E. coli AlkB in B (50). The secondary structure elements that differ between ABH8 and its homologs are colored in magenta in B and C.