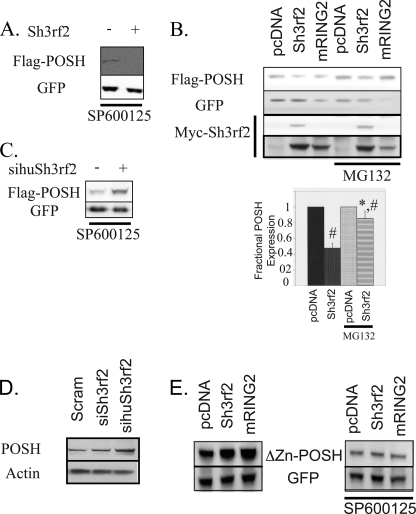
FIGURE 5.
Sh3rf2 reduces POSH levels under basal conditions by a proteasome-dependent mechanism, which requires the RING domain of both proteins. A, FLAG-tagged, wild-type POSH in pCMS·EGFP was co-expressed in HEK293 cells with either Sh3rf2 in the pcDNA3.1 vector or pcDNA3.1 alone. Cells were collected 20 h later and subjected to immunoblotting for FLAG. Membranes were reprobed for GFP to confirm equal transfection. SP600125 (20 μm) was added to prevent JNK activation by overexpressed POSH and self-stabilization of POSH. B, FLAG-tagged POSH in pCMS·EGFP was expressed in HEK293 cells with the indicated cDNAs in the pcDNA3.1 vector. The experiment was again performed in the presence of SP600125 (20 μm), and MG132 (20 μm) was added as indicated 20 h after transfection. Cells were collected 6 h after the addition of MG132. Equal quantities of cell lysate were subjected to immunoblotting for FLAG. Membranes were reprobed for GFP to confirm equal transfection efficiency and loading. Membranes were then reprobed for Myc to determine expression of tagged Sh3rf2. The lower panel shows a darker exposure of the Myc blot to show expression levels of the RING mutant. The graph at the bottom shows the results from three consecutive experiments. Band intensity in each case was determined, with POSH expression in the presence of pcDNA being set to 1. EGFP intensity was used to control for transfection efficiency and loading. The marked reduction in GFP in lanes containing RING2 is due to enhanced cell death in these cells. C, FLAG-tagged, wild-type POSH in pCMS-EGFP was expressed in HEK293 cells. Cells were treated with siRNA targeting human Sh3rf2 (sihuSh3rf2) or control siRNA in the presence of SP600125 (20 μm). Cells were collected 20 h later and subjected to immunoblot for FLAG. Membranes were reprobed for GFP to confirm equal transfection. D, HEK293 cells were treated with the indicated synthetic siRNAs in the presence of SP600125 (20 μm) for 20 h. Equal quantities of lysates were subjected to immunoblot for endogenous POSH. Membranes were reprobed for β-actin to ensure equal loading. E, Myc-tagged POSH lacking the RING domain (ΔZn-POSH) in pCMS-EGFP was co-expressed with the indicated cDNAs in the pcDNA3.1 vector in the presence or absence of SP600125 (20 μm). Lysates were collected 20 h later and subjected to Western immunoblot as indicated. Membranes were reprobed with anti-GFP to confirm equal loading and transfection.