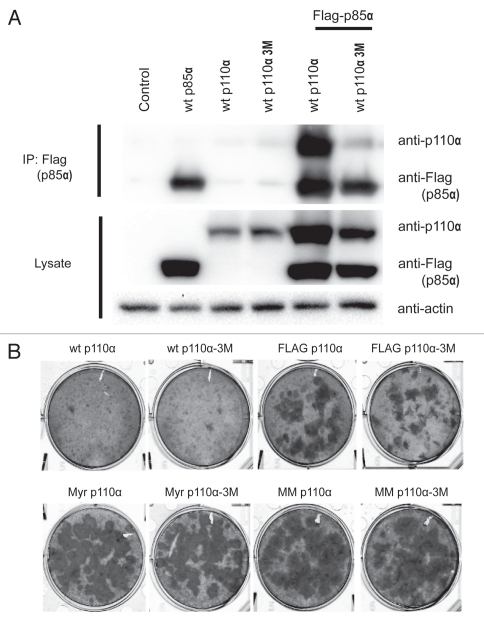
Figure 6.
Binding to p85 is not required for oncogenic transformation induced by NTT p110α. (A) Binding of p85a by p110α is greatly reduced by three point mutations (E23R, P27E and F95A referred to as 3M) in the adapter-binding domain. HEK293T cells were transfected using lipofectamine with empty pCAGGS vector (control lane) and Flag-p85a, wt p110α, p110α-3M alone or in combination as indicated. Forty-eight hours post transfection, the cells were lysed, and p85a was immunoprecipitated with Flag antibody-conjungated agarose beads. The precipitates were separated by SDS-PAGE and immunoblotted with anti-p110α and anti-Flag antibodies. Separately, protein gel blots of the cell lysates were prepared with anti-p110α and anti-Flag antibodies to reveal the total amounts of p110α and p85a. Actin was used as loading control. Substitution of NTT constructs for p110α yielded similar results. (B) Focus assays show that interrupting p85 binding does not affect the oncogenic activity of NTT constructs.