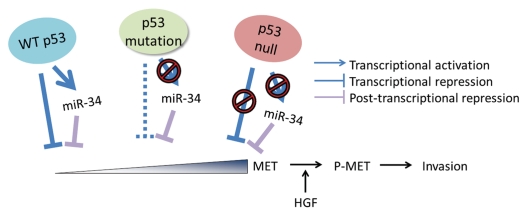
Figure 5.
Proposed model of MET-dependent cancer invasion preprogrammed by early alterations of p53-regulated feedforward loop and triggered by stromal cell-derived HGF. Feedforward loop regulation of MET by wild-type p53 consists of miR-34-dependent and -independent mechanisms. p53 protein with point mutations in its p53 DNA binding domain is unable to transactivate MET targeting miR-34. However, it still binds SP1, thereby repressing MET promoter, albeit to a limited extent. In contrast, lack of p53 due to null mutations results in incapacitations of both miR-34 transactivation and MET promoter repression, thereby leading to highest levels of MET. Although p53 mutations result in increased expression of MET, cells become highly motile and invasive only after MET activation by phosphorylation (P-MET). This phosphorylation is triggered by MET ligand HGF produced by cells of newly forming desmoplastic stroma. Thus, while alterations in p53/miR-34/MET network preprogram cells for increased motility and invasion, microenvironment may play a crucial role in triggering those properties during cancer progression.