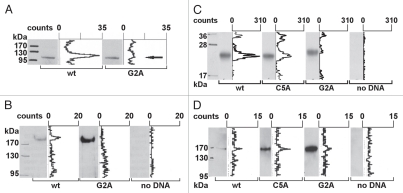
Figure 2.
Transcription factor NFAT5a is N-terminal myristoylated and palmitoylated. (A) In vitro transcription/translation (TNT) of NFAT5a(AA1–541)-GST (wt) and its G2A mutant was performed in the presence of3H-labeled myristate. Expression was monitored by protein gel blot detection.3H incorporation was measured with a TLC linear analyzer. The scan shows that only NFAT5a-GST with an intact glycine at position 2 incorporates the myristoyl anchor. (B) HeLa cells were transiently transfected with NFAT5a-HA (wt) and NFAT5a(G2A)-HA. Proteins were purified from cells after in vivo labeling with3H-myristate, detected by immunoblotting and scanned for3H incorporation. Although the western signal is significantly lower for wt NFAT5a-HA in comparison to the mutant, only wt gives rise to a radioactive signal. As a control, non-transfected cells were used, and no signal could be detected. (C) In vitro TNT of NFAT5a(AA1–123)-HA (wt) and its mutants C5A and G2A was performed in the presence of activated3H-palmitate-CoA. Incorporation of radioactive palmitate was monitored by TLC scanning. Immunoblotting was used to monitor protein expression. The western bands are comparable in strength. The TLC signal for the wt is about double the size of the C5A mutant's signal. There is no TLC signal for the G2A mutant. No plasmid was used for the control reaction. (D) HeLa cells were transiently transfected with NFAT5a-HA (wt) and its mutants C5A and G2A. After transfection, cells were subjected to in vivo labeling with3H-palmitate. Proteins were immunoprecipitated, quantified by immunoblotting and scanned for labeled palmitate attachment. Although the expression of the wt is significantly weaker than the mutant ones, the TLC signal strength is comparable. Non-transfected cells were used as a background control.