Throughout evolution, cells have developed diverse biological mechanisms to efficiently respond to changes in the extracellular stimuli. One such mechanism is a feedback loop. By feeding back a part of the output, the feedback loop permits the modulation of the level of output either by increasing (i.e., positive feedback) or decreasing (i.e., negative feedback) the level of input. This mechanism is widely employed in various signaling pathways to maintain the steady-state physiological condition known as homeostasis.
Recent studies have demonstrated the existence of an under-appreciated control system that conveys an input signal in a pre-determined way, regardless of how the output responds. In this case, the flow of events occurs only in the forward direction—from the input to the output. A type of this forward control system, called feed-forward loop, in which “A” turns on “B” and the action of “A” and “B” is integrated to turn on “C,” has been observed in transcriptional regulatory networks in yeast and bacteria and in the metabolic network of glycolysis in human erythrocytes.1–5 Whether other intracellular processes also exploit a feedforward cooperativity remains to be further investigated.
Mammalian polo-like kinase 1 (Plk1) belongs to the conserved polo subfamily of Ser/Thr protein kinases that is critically required for proper M-phase progression (reviewed in ref. 6). The polo-box domain (PBD) present in the C-terminal noncatalytic region is central for targeting the catalytic activity of Plk1 to specific subcellular structures (reviewed in ref. 7). PBD forms a phospho-Ser/Thr-binding module and binds to a phospho-epitope frequently generated either by Cdk1 or other Pro-directed kinases or by Plk1 itself (reviewed in ref. 7). Studies have shown that Plk1 PBD binds to the phospho-T78 (p-T78) motif of a centromere component PBIP1 (also named MLF1IP/KLIP1/CENP-50/CENP-U),8 and that this interaction is critical for its localization to late interphase centromeres and early mitotic kinetochores. Loss of this interaction results in insufficient recruitment of Plk1 to these sites, ultimately leading to mitotic block, chromosome missegregation and apoptotic cell death.8 Interestingly, Plk1 itself generates the T78 motif of PBIP1, thus promoting its own recruitment to the PBIP1-loaded centromeres/kinetochores through a mechanism called “self-priming and binding.”8,9 This mechanism is in contrast with the “nonself-priming and binding” mechanism, whose PBD-binding site is generated by another kinase, such as Pro-directed kinases.
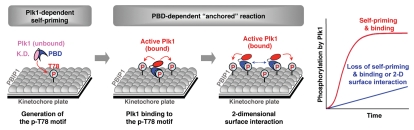
Further investigation of the Plk1-PBIP1 interaction revealed that PBD-dependent docking to the p-T78 motif of PBIP1 enables the catalytic activity of Plk1 to cooperatively phosphorylate the T78 residues of neighboring PBIP1 molecules bound to a “two-dimensional” surface (e.g., PBIP1 molecules restricted to the surface of a centromere/kinetochore plate rather than being present in the three-dimensional cytoplasm/nucleoplasm).10 Strikingly, either loss of PBD-dependent Plk1-PBIP1 interaction or lack of a sufficient number of surface-restricted PBIP1 molecules prevented Plk1 from cooperatively phosphorylating PBIP1.10 Thus, Plk1 utilized its own reaction product, p-T78-bearing PBIP1, to anchor itself and phosphorylate neighboring PBIP1 molecules in a feedforward manner (Fig. 1). Since PBD-dependent binding to the p-T78 motifs restricts Plk1 to the site of interactions (e.g., PBIP1-localized centromere/kinetochore plate), generation of additional p-T78-containing PBIP1 molecules allows Plk1 to carry out “two-dimensional surface interactions” with the former to further increase the efficiency of Plk1-dependent PBIP1 phosphorylation at this site (Fig. 1). The cooperative nature of the product-activated, feedforward mechanism underlying the formation of the Plk1-PBIP1 complex helps explain the ability of activated Plk1 to rapidly recruit itself to early mitotic kinetochores. Enhanced reaction kinetics with surface-restricted substrates was initially described with lipid bilayer-associated enzymes.11
Figure 1.
Schematic illustration of a product-activated, feedforward cooperativity in Plk1-dependent PBIP1 phosphorylation at a kinetochore plate. Unbound Plk1 stochastically interacts with kinetochore-loaded PBIP1 and phosphorylates the latter at T78 to generate a PBD-binding site. Once recruited to a kinetochore plate through the interaction with its reaction product, p-T78 PBIP1 (i.e., self-priming and binding), Plk1 then efficiently generates additional p-T78 epitopes on other PBIP1 molecules located proximally from the initial PBD-anchored site (i.e., PBD-dependent anchored reaction). The appearance of additional p-T78-containing PBIP1 molecules then permits Plk1 to perform “two-dimensional surface interactions” (dotted bidirectional arrow) with the former on the kinetochore plate and to phosphorylate even distantly placed PBIP1 molecules with an increased reaction efficiency. These sequential steps ensure a product-activated, feedforward cooperativity in Plk1-dependent phosphorylation of kinetochore-confined PBIP1 molecules, thus resulting in rapid recruitment of Plk1 to the PBIP1-localized kineotchores. Loss of self-priming and binding or lack of two-dimensional (2D) surface interactions due to insufficient surface-restricted PBIP1 molecules annihilates the cooperativity. Dotted Plk1 proteins on the third kinetochore plate denote one molecule of Plk1 carrying out surface interactions with neighboring p-T78 motifs.
At present, whether or how the above-described product-activated, feedforward process is regulated is not known. One possibility is that activation of a pathway(s) leading to the downregulation of Plk1 activity would decelerate this process. Alternatively, action of a phosphatase(s) that dephosphorylates the p-T78 of PBIP1 would directly antagonize Plk1-dependent PBIP1 phosphorylation and subsequent binding. Further investigation is necessary to better comprehend the mechanisms regulating this unusual self-propelled biological process.
Protein phosphorylation by kinases is widely used to regulate various intracellular events. Among the members of kinases, Plk1 is unique in that its catalytic activity functions in conjunction with a cis-acting phospho-binding PBD that mediates a product-activated, feedforward cooperativity at centromere/kinetochore plates. Given that the number of Plk1 substrates following the self-priming and binding mechanism continues to grow,7 and that Plk1 distinctly localizes to surface-confined structures, such as centrosomes, kinetochores and the midbody, the PBD-mediated feedforward mechanism is likely a fundamental biochemical process that ensures a rapid induction of Plk1-dependent events at specific subcellular locations. Further studies aimed at understanding the molecular mechanisms by which Plk1 promotes various intracellular processes may prove to be an important challenge for years to come.
Comment on: Park JE, et al. Proc Natl Acad Sci USA. 2011;108:8200–8205. doi: 10.1073/pnas.1102020108.
References
- 1.Bali M, et al. CR Acad Sci III. 2001;324:185–199. doi: 10.1016/s0764-4469(00)01295-6. [DOI] [PubMed] [Google Scholar]
- 2.Hayot F, et al. J Theor Biol. 2005;234:133–143. doi: 10.1016/j.jtbi.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Ratushny AV, et al. Biophys J. 2008;95:3715–3723. doi: 10.1529/biophysj.108.134064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mangan S, et al. Proc Natl Acad Sci USA. 2003;100:11980–11985. doi: 10.1073/pnas.2133841100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangan S, et al. J Mol Biol. 2003;334:197–204. doi: 10.1016/j.jmb.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 6.Archambault V, et al. Nat Rev Mol Cell Biol. 2009;10:265–275. doi: 10.1038/nrm2653. [DOI] [PubMed] [Google Scholar]
- 7.Park JE, et al. Cell Mol Life Sci. 2010;67:1957–1970. doi: 10.1007/s00018-010-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang YH, et al. Mol Cell. 2006;24:409–422. doi: 10.1016/j.molcel.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Lee KS, et al. Cell Cycle. 2008;7:141–145. doi: 10.4161/cc.7.2.5272. [DOI] [PubMed] [Google Scholar]
- 10.Park JE, et al. Proc Natl Acad Sci USA. 2011;108:8200–8205. doi: 10.1073/pnas.1102020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carman GM, et al. J Biol Chem. 1995;270:18711–18714. doi: 10.1074/jbc.270.32.18711. [DOI] [PubMed] [Google Scholar]