Figure 5.
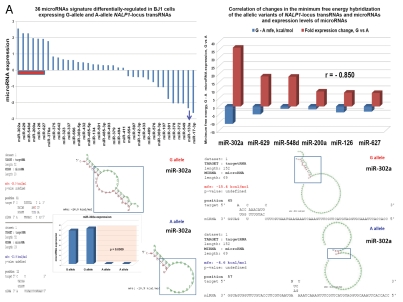
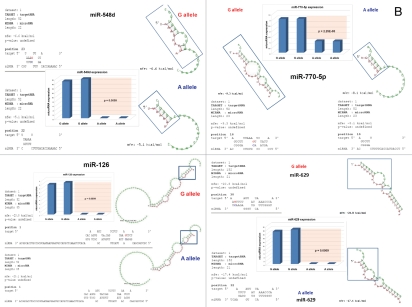
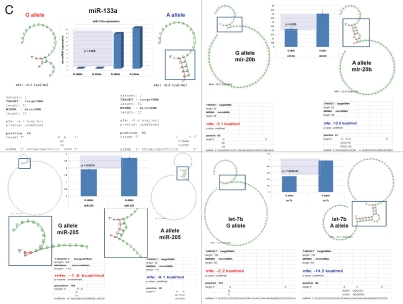
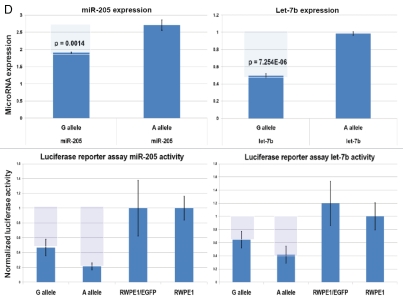
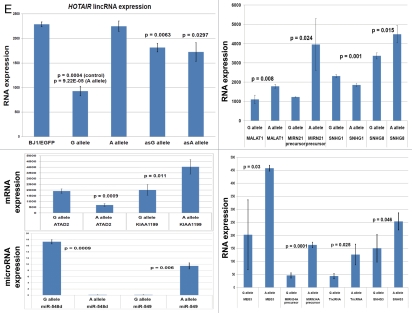
snpRNA sequences represent allele-specific “decoy” targets of microRNAs that function as SNP allele-specific enhancers of microRNA expression and activity. Experimental evidence and computational analysis supporting the allele affinity model of snpRNA-mediated regulation of microRNA expression and activity. ABI TaqMan PCR-based screen identifies a statistically significant set of 36 microRNAs, expression of which is (1) altered at least 1.5-fold in NLRP1-locus snpRNA-expressing cells compared to control BJ1/EGFP cells and (2) differentially regulated in pathology-linked G allele-expressing BJ1 cells compared to the ancestral A allele-expressing cells (set of bars in the top left panel). Note that 18 of 36 (50%) of these microRNAs are derived from the single microRNA cluster on the ∼ 200 kb continuous region of 14q32 band of chromosome 14, which suggests that 14q32 cluster microRNAs may be primary molecular targets of the NLRP1-locus snpRNAs (Fig. S6). Statistically highly significant inverse correlation between allele-specific changes in minimal free energy (mfe) of snpRNA/microRNA hybridization and experimentally defined changes of microRNA expression; that is, lower mfe values correspond to higher levels of microRNA expression (set of bars in the top right panel). Experimental evidence and computational analysis supporting the allele affinity model of snpRNA-mediated regulation of microRNA expression and activity. Expression of high affinity (low mfe) snpRNA alleles in human cells (defined by allele-specific changes in minimal free energy of snpRNA/microRNA hybridization) is associated with increase in abundance levels of corresponding microRNAs. These relationships are shown for individual microRNAs, the abundance levels of which in human cells are induced [miR-302a; miR-629; miR-548d; miR-126; miR-770-5p] by forced expression of pathology-linked G-allele snpRNAs compared to ancestral A allele-expressing cells. Inset bars show the results of Q-PCR analysis of expression of corresponding microRNAs (see additional analysis and examples in the Sup. Material and Fig. S8). Experimental evidence and computational analysis supporting the allele affinity model of snpRNA-mediated regulation of microRNA expression and activity. Expression of high affinity (low mfe) snpRNA alleles in human cells (defined by allele-specific changes in minimal free energy of snpRNA/microRNA hybridization) is associated with increase in abundance levels of corresponding microRNAs. These relationships are shown for individual microRNAs, the abundance levels of which in human cells are repressed [miR-20b; let-7b; miR-133a; miR-205] by forced expression of pathology-linked G-allele snpRNAs compared to ancestral A allele-expressing cells. Inset bars show the results of Q-PCR analysis of expression of corresponding microRNAs (see additional analysis and examples in the Sup. Material and Fig. S8). Experimental evidence and computational analysis supporting the allele affinity model of snpRNA-mediated regulation of microRNA expression and activity. Expression of NLRP1-locus snpRNAs in human cells induces allele sequence-dependent changes in expression (top sets of bars) and activity (bottom sets of bars) of microRNAs. Q-PCR measurements of the expression levels (top sets of bars) and luciferase reporter assay of miR-205 and let-7b activities (bottom sets of bars) in RWPE1 cells stably expressing distinct allelic variants of the NLRP1-locus snpRNAs demonstrate significant changes of expression and activity of both microRNAs in high-affinity (A allele) snpRNA-expressing cells compared to low affinity (G allele) snpRNA allele-expressing cells. Experimental evidence and computational analysis supporting the allele affinity model of snpRNA-mediated regulation of microRNA expression and activity. Microarray analysis of human BJ1 cells engineered to stably express distinct allelic variants of the NLRP1-locus transRNAs reveals allele-specific alterations of expression levels of multiple distinct classes of noncoding RNAs which include snoRNAs and snoRNA host genes (SNORD113; SNHG1; SNHG3; SNHG8), long noncoding RNAs (MEG3, tncRNA, HOTAIR, and MALAT1), microRNAs, microRNA precursors, and proteincoding microRNA host genes (ATAD2; KIAA1199). Note that changes of expression of intron-residing microRNAs miR-548d (intron of the ATAD2 gene) and miR-549 (intron of the KIAA1199 gene) are in good correspondence with allele-specific expression levels of corresponding microRNA host genes, which suggests a coordinated mechanism of regulation (see also Fig. S6).