Abstract
Study Objectives:
To determine the effects of bilevel positive airway pressure with pressure release technology (Bi-Flex) on adherence and efficacy in children and adolescents compared to standard continuous positive airway pressure (CPAP) therapy. We hypothesized that Bi-Flex would result in improved adherence but similar efficacy to CPAP.
Methods:
This was a randomized, double-blinded clinical trial. Patients with obstructive sleep apnea were randomized to CPAP or Bi-Flex. Repeat polysomnography was performed on pressure at 3 months. Objective adherence data were obtained at 1 and 3 months.
Results:
56 children and adolescents were evaluated. There were no significant differences in the number of nights the device was turned on, or the mean number of minutes used at pressure per night for CPAP vs Bi-Flex (24 ± 6 vs 22 ± 9 nights, and 201 ± 135 vs 185 ± 165 min, respectively, for Month 1). The apnea hypopnea index decreased significantly from 22 ± 21/h to 2 ± 3/h on CPAP (p = 0.005), and 18 ± 15/h to 2 ± 2/h on Bi-Flex (p < 0.0005), but there was no significant difference between groups (p = 0.82 for CPAP vs Bi-Flex). The Epworth Sleepiness Scale decreased from 8 ± 5 to 6 ± 3 on CPAP (p = 0.14), and 10 ± 6 to 5 ± 5 on Bi-Flex (p < 0.0005; p = 0.12 for CPAP vs Bi-Flex).
Conclusions:
Both CPAP and Bi-Flex are efficacious in treating children and adolescents with OSAS. However, adherence is suboptimal with both methods. Further research is required to determine ways to improve adherence in the pediatric population.
Citation:
Marcus CL; Beck SE; Traylor J; Cornaglia MA; Meltzer LJ; DiFeo N; Karamessinis LR; Samuel J; Falvo J; DiMaria M; Gallagher PR; Beris H; Menello MK. Randomized, double-blind clinical trial of two different modes of positive airway pressure therapy on adherence and efficacy in children. J Clin Sleep Med 2012;8(1):37-42.
Keywords: CPAP, obstructive sleep apnea, Bi-Flex
A denotonsillectomy is the primary treatment for the childhood obstructive sleep apnea syndrome (OSAS).1 However, for children with additional medical conditions such as craniofacial anomalies or obesity, or those with persistent OSAS following surgery, positive airway pressure (PAP) therapy is often required. Previous studies using objective adherence monitoring have shown suboptimal adherence to PAP in children.2–4
A number of novel methodologies have been developed to increase patient comfort, and hence adherence, with PAP. Bilevel pressure (BPAP) may feel more comfortable to patients, although it has not been shown to increase adherence in either adults5 or children2 with OSAS. Nevertheless, the American Academy of Sleep Medicine clinical guidelines recommend BPAP for patients uncomfortable on or intolerant of CPAP.6 Other investigators have evaluated the use of pressure relief technologies to more closely mimic natural breathing patterns. The C-Flex technology (Philips Respironics) results in a slight decrease in pressure during early expiration, whereas Bi-Flex (Philips Respironics) results in pressure decrements during both late inspiration and expiration. The effects of these technologies remain controversial. Some studies in adults have shown improved comfort or adherence with C-Flex or Bi-Flex,7,8 whereas other studies have not shown significant improvements.9–12
BRIEF SUMMARY
Current Knowledge/Study Rationale: Children with the obstructive sleep apnea syndrome often have poor adherence to positive airway pressure (PAP) therapy. Some, but not all, studies have shown improved adherence to PAP in adults using pressure relief technologies; however, this technology has not been evaluated in children.
Study Impact: This study has shown that PAP use results in improved polysomnographic parameters of OSAS and decreased daytime sleepiness in children, although adherence in this population is suboptimal. However, there was no improvement in adherence or efficacy using bilevel pressure relief technology compared to continuous positive airway pressure.
In children, factors influencing PAP adherence are likely different from those in adults. Furthermore, as children have lower tidal volumes and faster respiratory rates than adults; they do not always have adequate triggering and cycling of noninvasive ventilation.13 It is therefore important that new technologies be evaluated in children before they are used clinically.
We hypothesized that the use of Bi-Flex would be associated with improved adherence and similar efficacy compared to CPAP in children with OSAS. We elected to compare Bi-Flex to CPAP in this study, rather than comparing Bi-Flex to BPAP, as theoretically Bi-Flex would be the most comfortable mode of ventilation, whereas CPAP is the clinical standard of care and is the cheapest PAP option. We therefore performed a randomized, double-blinded study comparing CPAP to Bi-Flex in children with OSAS.
METHODS
Study Group
Consecutive children with OSAS due to any cause, aged 2-16 years, who were naïve to PAP and who were thought clinically to require PAP, were eligible. The majority had undergone prior adenotonsillectomy but had additional risk factors for OSAS such as obesity or genetic syndromes. Subjects were classified as obese if their body mass index was ≥ 95th percentile for age and sex.14
Protocol
All subjects had undergone a baseline clinical polysomnogram prior to study entry. Subjects were fitted with a PAP mask and allowed to habituate to the mask for 2 weeks.2 They then underwent a PAP titration study, randomized to either CPAP or Bi-Flex. PAP is used less in children than adults, thereby limiting study samples. As data were already available in the literature regarding adherence with CPAP in children, subjects were randomized in a 3:1 fashion (3 Bi-Flex to 1 CPAP). Both subjects and study investigators were blinded to the mode used. The study was performed by a sleep technologist who by necessity was not blinded but who performed the titration according to a fixed protocol. Studies were initiated on pressure levels of 4 cm H2O for those randomized to CPAP, and 8/4 cm H2O for those randomized to Bi-Flex. Pressures were then increased by 2 cm H2O as needed for obstructive apneas and hypopneas. Titration polysomnograms were reviewed by a separate pediatric sleep physician (SB) who was not blinded but played no other role in the study. The research coordinator, statistician, and other investigators were blinded to the mode used. The PAP equipment was modified by Philips Respironics so that the 2 machines looked identical and there was no digital display of pressure settings or mode. Subjects received free PAP equipment, including heated humidification, interfaces, and tubing, the morning after the titration polysomnogram.
A phone call was made 48 h after initiation to assess use, provide support, and identify and troubleshoot side effects and barriers to use, such as nasal congestion, eye irritation, skin breakdown, equipment problems, or behavioral issues. Nasal symptoms were treated as clinically appropriate with saline, nasal steroids, antihistamines, and/or montelukast. Behavioral modification support was provided as needed.15 Another phone call was made at 2 weeks to assess the same parameters and to provide positive reinforcement. Subjects were seen on a monthly basis, and objective adherence data were downloaded using EncorePro 2 software. At 3 months, a repeat polysomnogram was performed with the subject on the prescribed pressure; pressure levels were adjusted further if needed. Subjects were provided free equipment at the end of the study.
Subjects and their caregivers completed a number of questionnaires including the Epworth Sleepiness Scale modified for children,16 and the Nasal Obstruction Symptom Evaluation (NOSE).17 Other questionnaire data will be reported separately.
The study was approved by the Internal Review Board at The Children's Hospital of Philadelphia. Written informed consent was obtained from the parent or legal guardian, and assent from children 7 years of age or older. The study was registered at Clinicaltrials.gov (#NCT00458406).
Data Analysis
The primary adherence outcome measure was the mean number of minutes used per night at pressure for Month 1. We also evaluated the number of nights that the machine was turned on during Month 1. In addition, as some children require gradual habituation to PAP,15 we evaluated use (both mean number of minutes used at pressure per night and number of nights used) at Month 3. We also evaluated the time that the machine was turned on (but was not necessarily providing full pressure) per night for Month 1. This was done because we thought that children were more likely to have leaks than older patients due to decreased availability of appropriately sized pediatric interfaces; young children were likely to pull off the mask during sleep without the parents necessarily being aware; and children were likely to sleep through mask displacement events, as children have a high arousal threshold.18 Adherence data were analyzed continuously rather than categorically, as the ideal cutoff for PAP adherence in children is not known. For adults, adherence of 4 h/night has arbitrarily been considered to be adequate19; however, children sleep longer than adults and would likely benefit from longer PAP use.
Unless otherwise specified, data are shown as mean ± SD. Histograms and Kolmogorov-Smirnov tests were used to examine normalcy of distribution. Logarithmic transformations were applied to skewed data prior to statistical testing; in the event that the data could not be normalized, nonparametric tests were used. Differences between subjects under 2 conditions were analyzed using paired t-tests or rank sum tests, and differences between groups were analyzed using independent t-tests or signed rank tests. Pearson or Spearman correlation coefficients were used to examine associations between various outcomes.
The 95% confidence intervals (CI) of the mean differences between groups were constructed. For outcomes with skewed distributions, median differences and Hodges-Lehmann 95% CI for the differences between population medians were calculated.20 Observed effect sizes were also calculated. For nonparametric tests, observed effect sizes were calculated based on rank-transformed data. Effect sizes were then calculated by dividing the difference between the mean ranks of the 2 groups by the average SD of the ranks within the 2 groups. These Cohen-type estimates have been found to be unbiased for normally distributed variables, on average giving the same effect sizes as analyses of untransformed normally distributed variables.21 In addition, an alternative effect size index was calculated based on a generalization of the Mann-Whitney U statistic. This effect size measure has been labeled a probabilistic index, and provides an intuitive and simple effect size estimate for nonparametric outcome22; the formula is [2(U/mn) −1], where U/mn is the Mann-Whitney U statistic divided by the product of the 2 sample sizes.23 The resulting probabilistic index is an indication of the incremental probability of observing, for a randomly chosen subject, a higher outcome value for a Bi-Flex subject relative to a CPAP subject.
P values < 0.05 were considered significant.
Sample Size Determination
The study sample size of 45 in the Bi-Flex group and 15 in the CPAP group had 80% power to detect an effect size ≥ 0.85 with a 0.05 two-sided significance level. In addition, with this sample size, the 95% CI for the difference between the 2 means had a range of 1.282 SD.
RESULTS
Study Group
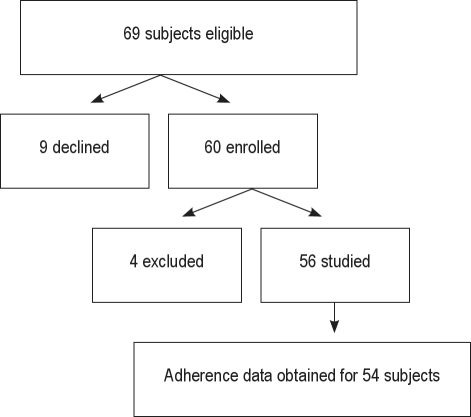
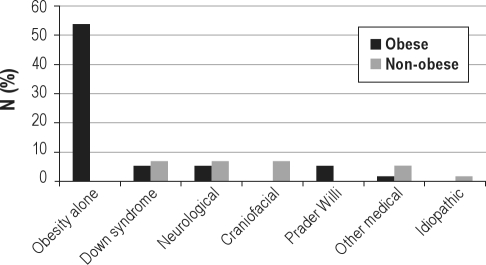
Sixty-nine consecutive families were approached for the study. Nine families declined the study: 2 because of distance, 5 because of the burden of the study visits, 1 for no stated reason, and 1 because of severe parental illness. Thus, 60 children were enrolled. Four children were subsequently excluded. One child with Down syndrome developed pneumonia and adult respiratory distress syndrome one week after enrollment and underwent tracheotomy and long-term mechanical ventilation; one child with nasopharyngeal stenosis underwent surgery; one adolescent was institutionalized without his PAP equipment; and one child moved away after consenting but before entering the study. Thus, data are presented on 56 subjects (Figure 1). Subject characteristics are shown in Table 1. As expected based on the pathophysiology of pediatric OSAS, many children were obese and almost half had underlying major medical problems such as craniofacial conditions, genetic syndromes or neurologic disease (Table 1 and Figure 2).
Figure 1.
Flow diagram of subject enrollment
Table 1.
Study group
| CPAP | Bi-Flex | |
|---|---|---|
| N | 13 | 43 |
| Age (yr) | 12 ± 4 | 12 ± 4 |
| Males | 10 (77) | 28 (65) |
| Race | ||
| African American | 6 (46) | 27 (63) |
| Caucasian | 6 (46) | 14 (33) |
| More than one race | 1 (8) | 2 (5) |
| Hispanic ethnicity | 2 (15) | 3 (7) |
| BMI z-score | 2.1 ± 0.8 | 2.1 ± 1.0 |
| Obese | 9 (69) | 30 (70) |
| Presence of major medical conditions | 5 (39) | 20 (47) |
Data shown as mean ± SD or N (%). There were no significant differences between groups.
Figure 2.
The percentage of obese and non-obese subjects with varying medical conditions are shown
A total of 5 subjects did not complete all aspects of the study. One of these subjects declined the 3-month polysomnogram but completed all other portions of the study. The other 4 subjects were lost to follow-up; however, 2 of these subsequently returned for clinical follow-up, and hence 3-month downloads were able to be obtained. Thus, Month 3 adherence data were available for all but 2 subjects (one in each group). Month 1 adherence data could not be downloaded for 2 children due to equipment malfunction; however, Month 3 data were available for both of these children.
Adherence
Adherence data are shown in Tables 2-3 and Figure 2. There was a large variability in adherence. Most subjects attempted to use PAP on most nights, with the devices being turned on more than two-thirds of the nights for Month 1. Only 2 subjects (one in each group) did not turn the machine on at all in Month 1. However, average nightly use varied widely between subjects (Table 2), ranging from 1-536 min/night for Month 1. The average number of minutes that the machine was turned on per night in Month 1 was much higher than the mean use at pressure, with the difference averaging > 100 min per night (107 ± 118 min, p < 0.0005). There were no differences in any of the adherence parameters between the 2 groups.
Table 2.
Adherence data
| CPAP | Bi-Flex | |
|---|---|---|
| Number of nights used, Month 1 | 24 ± 6 | 22 ± 9 |
| Number of nights used, Month 3 | 18 ± 10 | 19 ± 9 |
| Mean number of minutes turned on, Month 1 | 299 ± 120 | 294 ± 176 |
| Mean number of minutes used at pressure, Month 1 | 201 ± 135 | 185 ± 165 |
| Mean number of minutes used at pressure, Month 3 | 125 ± 147 | 183 ± 169 |
Data shown as mean ± SD. There were no significant differences between groups.
Table 3A.
Observed effect size, standard error (SE), and 95% CI of the mean differences for key normally distributed data
| Outcome | CPAP (mean ± SD) | Bi-Flex (mean ± SD) | t-test p value | Effect size* | Mean difference | SE of difference | 95% CI of differenc |
|---|---|---|---|---|---|---|---|
| Number of minutes used at pressure, Month 1 | 201 ± 135 | 185 ± 165 | 0.76 | 0.10 | −16 | 52 | −121, 89 |
| △Epworth Scale | −2.3 ± 4.8 | −4.5 ± 4.0 | 0.12 | 0.46 | −2.2 | 1.4 | −5.0, 0.6 |
The difference between the 2 groups' means divided by their common SD.
Table 3B.
Observed effect size (based on rank-transformed data) and Hodges-Lehmann 95% CI for the difference between population medians for nonparametric data
| Outcome | CPAP median (min, max) | Bi-Flex median (min, max) | Mann-Whitney p value | Effect size* | Probabilistic index** | Observed median difference | Population median difference | 95% CI of population difference |
|---|---|---|---|---|---|---|---|---|
| Number of nights used, Month 1 | 26 (13, 31) | 27 (1, 31) | 0.93 | 0.03 | 0.49 | 1.0 | 0.0 | −5, 4 |
| △AHI | −13.7 (−42.8, 6.4) | −8.8 (−62.1, 5.1) | 0.82 | 0.07 | 0.48 | 4.9 | 0.5 | −10.3, 8.1 |
AHI, apnea hypopnea index.
Based on rank-transformed data. The difference between the 2 groups' mean ranks divided by the average SD of their ranks.
Probabilistic Index = [2(U/mn) −1], where U/mnis the Mann-Whitney Ustatistic divided by the product of the 2 sample sizes.
PAP use declined over time in both groups (for nights used, p = 0.030 for CPAP and 0.006 for Bi-Flex; for mean number of minutes used at pressure per night, p = 0.045 for CPAP and 0.598 for Bi-Flex). There was no significant difference in the decline in usage between the 2 modes.
Many of the study subjects were obese. About half of the subjects had obesity alone, approximately another 20% had obesity associated with other medical conditions such as Down syndrome, Prader Willi syndrome, or spina bifida (Figure 2). There were no significant differences in any of the adherence outcomes between the obese and non-obese subjects, and no interactions between PAP modes by obesity status on adherence outcomes.
Efficacy
Polysomnographic parameters at the beginning and end of the study are shown in Table 4. All subjects had adequate control of their OSAS by PAP on the titration night, with highly significant improvements in respiratory parameters compared to baseline. There were no differences in these improvements between the 2 treatment modalities.
Table 4.
Polysomnographic and clinical data
| CPAP |
Bi-Flex |
|||
|---|---|---|---|---|
| Baseline | Final titration | Baseline | Final titration | |
| N | 13 | 12 | 43 | 39 |
| Total sleep time (min) | 392 ± 119 | 422 ± 44 | 419 ± 76 | 416 ± 102 |
| Sleep efficiency (%) | 84 ± 8 | 85 ± 7 | 81 ± 12 | 83 ± 20 |
| REM sleep (% total sleep time) | 17 ± 9 | 22 ± 6 | 20 ± 6 | 23 ± 8a |
| Arousal index (N/h) | 25 ± 20 | 14 ± 8b | 22 ± 13 | 17 ± 20c |
| Apnea hypopnea index (N/h) | 22 ± 21 | 2 ± 3d | 18 ± 15 | 2 ± 2c |
| SpO2nadir (%) | 81 ± 12 | 92 ± 3e | 78 ± 13 | 90 ± 4c |
| Peak end-tidal CO2(mm Hg) | 56 ± 3 | 55 ± 4 | 57 ± 6 | 55 ± 5f |
| Epworth Sleepiness Scale | 8 ± 5 | 6 ± 3 | 10 ± 6 | 5 ± 5c |
| NOSE | 10 ± 6 | 4 ± 5g | 7 ± 6 | 5 ± 5 |
NOSE, Nasal Obstruction Symptom Evaluation. Data shown as mean ± SD. There were no significant differences in polysomnographic or clinical parameters between the 2 groups, either at baseline or final titration.
p value (baseline vs final titration) = 0.079.
p value (baseline vs final titration) = 0.012.
p value (baseline vs final titration) < 0.0005.
p value (baseline vs final titration) = 0.005.
p value (baseline vs final titration) = 0.008.
p value (baseline vs final titration) = 0.024.
p value (baseline vs final titration) = 0.007.
Initial pressures were 8 ± 2 cm H2O in the CPAP group, and 13 ± 3 cm H2O for inspiratory pressure and 7 ± 2 cm H2O for expiratory pressure in the Bi-Flex group. There was a small but significant increase in titrated pressure at the Month 3 polysomnogram in both groups; pressures increased to 10 ± 3 cm H2O (p = 0.031) in the CPAP group and 14 ± 3 (inspiratory) / 8 ± 3 (expiratory) cm H2O (p = 0.001) in the Bi-Flex group.
Clinical Response
There was no difference in the Epworth Sleepiness Scale or NOSE between the 2 groups at baseline. There was a significant decrease in the Epworth Scale at Month 3 in subjects receiving Bi-Flex (Table 4). In the CPAP group, there was a decline in the Epworth Scale score (Table 4) that did not reach statistical significance, possibly due to smaller sample size. There was no difference in the change in Epworth between CPAP vs Bi-Flex. The NOSE score decreased on PAP in both groups; this was significant in the CPAP group (Table 4).
Adverse Events
No subject experienced any serious complications from PAP. Minor complications included nasal symptoms, eye irritation, and skin breakdown.
Children Younger Than Seven Years of Age
As PAP is not approved by the Food and Drug Administration for children < 7 years of age or < 40 lb (18.2 kg) in weight, we additionally reviewed this subgroup of children separately. Nine (16%) of the children were younger than 7 years of age, and 4 (7%) weighed < 18.2 kg. Children < 7 years of age had similar efficacy (decrease in AHI from 17 ± 13/h to 1 ± 0.4/h, with a mean change of −17 ± 13/h) compared to older children (decrease in AHI from 19 ± 17/h to 2 ± 2/h, for a mean change of −16 ± 16/h) (NS). Paired tests indicated a significant difference between AHI at baseline and AHI at final titration for both younger and older children (p = 0.018 and p < 0.0005, respectively). Overall adherence in the young children was 188 ± 133 (range 3-410) minutes per night at month 1, which was not significantly different from the older children (188 ± 164, range 1-536).
DISCUSSION
This study has shown that both CPAP and Bi-Flex were efficacious in the treatment of children and adolescents with OSAS. Both modes resulted in major improvements in polysomnographic parameters. Subjective sleepiness, as measured by the Epworth Sleepiness Scale, improved with Bi-Flex and tended to improve with CPAP. However, contrary to our hypothesis, we did not find improved adherence with Bi-Flex compared to CPAP.
Children in this study had suboptimal CPAP adherence, despite receiving free equipment, behavioral counselling, and close follow-up and support. Several other studies have evaluated CPAP adherence in children using objective adherence data, and have also shown suboptimal adherence. A multicenter study of CPAP and bilevel pressure use found that adherence averaged only 3.8 ± 3.3 h per night, with no significant difference between the two modes.2 O'Donnell et al. found that only 65 of 79 potential pediatric CPAP patients initially accepted CPAP; of those 65, a further 17 children later declined CPAP.3 Of the 50 children in whom objective adherence data could be obtained, mean use was 4.7 h/night. Massa et al. reported successful long-term use of CPAP in only 40 of 66 children.24 In contrast to these studies, Uong et al. reported better adherence in a study population limited to children 7 years of age and older.4 Clearly, current CPAP treatment options for children and adolescents are inadequate as they are limited by poor adherence.
In order to improve adherence in PAP users, attempts have been made to improve patient comfort using novel techniques for pressure delivery. Several studies have evaluated the use of CPAP versus C-Flex or Bi-Flex in adults with OSAS, with mixed results. Two studies demonstrated improved adherence with either C-Flex or Bi-Flex compared to CPAP,7,8 whereas other studies found no difference in adherence10–12 or in patient satisfaction.9 Thus, the findings of the current study, which is the first to evaluate pressure relief technology in children, are similar to most of the adult studies in showing no definite advantage to using pressure relief technology over the cheaper alternatives. The failure to detect a difference in adherence between CPAP and Bi-Flex raises questions regarding power and sample size, and also invokes the distinction between statistical versus clinical significance. It has been suggested that a moderate effect size of 0.5, or equivalently, a “one-half standard deviation” rule, is clinically significant.25 The mean differences and observed effect sizes based on parametric tests of both raw and rank-transformed data found in this study were well below these general guidelines (Table 3), and suggest that any differences between the two groups were clinically insignificant.
Subjects spent a much greater time with the PAP device turned on then with the PAP device used at set pressure (Table 4). This illustrates the importance of managing airleaks in the pediatric population. In some cases, it may be appropriate to use low-pressure alarms to alert the caregiver that a leak is present or that the child has displaced the mask.
PAP is known to cause nasal symptoms. However, in the current study, nasal symptoms on PAP were decreased from baseline. This was probably due to the combination of heated humidity and aggressive medical treatment of nasal symptoms.26
PAP is not approved by the Food and Drug Administration for children younger than 7 years of age or weighing less than 40 pounds, although PAP interfaces have been approved down to age 2 years. Nevertheless, PAP is used widely in children of all ages2–4,24,27–29 as the alternative is often tracheotomy. In this study, children younger than 7 years of age had similar efficacy and adherence to older children, and no serious adverse events, suggesting that PAP use is safe and efficacious in young children.
In summary, this study has shown that Bi-Flex is as efficacious as CPAP in treating OSAS in children and adolescents, but does not result in improved adherence. Adherence was disappointingly low in the pediatric patients. Further research is required to determine ways to improve adherence, but these are unlikely to be related to technologic advances alone.
DISCLOSURE STATEMENT
This was an investigator-initiated (CLM) study funded by Philips Respironics. All data collection, statistical analyses and manuscript writing were performed by the investigators independent of Philips Respironics. Dr. Marcus receives research support from Philips Respironics. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the patients and their families who participated in this study, and all the sleep technologists at the Sleep Center of Children's Hospital of Philadelphia for their help with this study.
REFERENCES
- 1.American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:704–12. doi: 10.1542/peds.109.4.704. [DOI] [PubMed] [Google Scholar]
- 2.Marcus CL, Rosen G, Ward SL, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006;117:e442–51. doi: 10.1542/peds.2005-1634. [DOI] [PubMed] [Google Scholar]
- 3.O'Donnell AR, Bjornson CL, Bohn SG, Kirk VG. Compliance rates in children using noninvasive continuous positive airway pressure. Sleep. 2006;29:651–8. [PubMed] [Google Scholar]
- 4.Uong EC, Epperson M, Bathon SA, Jeffe DB. Adherence to nasal positive airway pressure therapy among school-aged children and adolescents with obstructive sleep apnea syndrome. Pediatrics. 2007;120:e1203–11. doi: 10.1542/peds.2006-2731. [DOI] [PubMed] [Google Scholar]
- 5.Reeves-Hoche MK, Hudgel DW, Meck R, Witteman R, Ross A, Zwillich CW. Continuous versus bilevel positive airway pressure for obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:443–9. doi: 10.1164/ajrccm.151.2.7842204. [DOI] [PubMed] [Google Scholar]
- 6.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157–71. [PMC free article] [PubMed] [Google Scholar]
- 7.Aloia MS, Stanchina M, Arnedt JT, Malhotra A, Millman RP. Treatment adherence and outcomes in flexible vs standard continuous positive airway pressure therapy. Chest. 2005;127:2085–93. doi: 10.1378/chest.127.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballard RD, Gay PC, Strollo PJ. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J Clin Sleep Med. 2007;3:706–12. [PMC free article] [PubMed] [Google Scholar]
- 9.Mulgrew AT, Cheema R, Fleetham J, Ryan CF, Ayas NT. Efficacy and patient satisfaction with autoadjusting CPAP with variable expiratory pressure vs standard CPAP: a two-night randomized crossover trial. Sleep Breath. 2007;11:31–7. doi: 10.1007/s11325-006-0078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilius G, Happel A, Domanski U, Ruhle KH. Pressure-relief continuous positive airway pressure vs constant continuous positive airway pressure: a comparison of efficacy and compliance. Chest. 2006;130:1018–24. doi: 10.1378/chest.130.4.1018. [DOI] [PubMed] [Google Scholar]
- 11.Pepin JL, Muir JF, Gentina T, et al. Pressure reduction during exhalation in sleep apnea patients treated by continuous positive airway pressure. Chest. 2009;136:490–7. doi: 10.1378/chest.08-2646. [DOI] [PubMed] [Google Scholar]
- 12.Bakker J, Campbell A, Neill A. Randomized controlled trial comparing flexible and continuous positive airway pressure delivery: effects on compliance, objective and subjective sleepiness and vigilance. Sleep. 2010;33:523–9. doi: 10.1093/sleep/33.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liner LH, Marcus CL. Ventilatory management of sleep-disordered breathing in children. Curr Opin Pediatr. 2006;18:272–6. doi: 10.1097/01.mop.0000193301.63259.84. [DOI] [PubMed] [Google Scholar]
- 14.Rosner B, Prineas R, Loggie J, Daniels SR. Percentiles for body mass index in U.S.children 5 to 17 years of age. J Pediatr. 1998;132:211–22. doi: 10.1016/s0022-3476(98)70434-2. [DOI] [PubMed] [Google Scholar]
- 15.Koontz KL, Slifer KJ, Cataldo MD, Marcus CL. Improving pediatric compliance with positive airway pressure therapy: the impact of behavioral intervention. Sleep. 2003;26:1010–5. doi: 10.1093/sleep/26.8.1010. [DOI] [PubMed] [Google Scholar]
- 16.Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114:768–75. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- 17.Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130:157–63. doi: 10.1016/j.otohns.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Busby KA, Mercier L, Pivik RT. Ontogenetic variations in auditory arousal threshold during sleep. Psychophysiology. 1994;31:182–8. doi: 10.1111/j.1469-8986.1994.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 19.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30:711–9. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodges JR, Lehmann EL. Estimates of location based on rank tests. Ann Math Stat. 1963;34:598–611. [Google Scholar]
- 21.Hopkins WG. Rank transformations: non-parametric models. A new view of statistics. 2000. http//www.sportsci.org/resource/stats/nonparms.html.
- 22.Acion L, Peterson JJ, Temple S, Arndt S. Probabilistic index: an intuitive non-parametric approach to measuring the size of treatment effects. Stat Med. 2006;25:591–602. doi: 10.1002/sim.2256. [DOI] [PubMed] [Google Scholar]
- 23.Chen M, Kianifard F. A nonparametric procedure associated with a clinically meaningful efficacy measure. Biostatistics. 2000;1:293–8. doi: 10.1093/biostatistics/1.3.293. [DOI] [PubMed] [Google Scholar]
- 24.Massa F, Gonsalez S, Laverty A, Wallis C, Lane R. The use of nasal continuous positive airway pressure to treat obstructive sleep apnoea. Arch Dis Child. 2002;87:438–43. doi: 10.1136/adc.87.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koziol JA, Feng AC. On the analysis and interpretation of outcome measures in stroke clinical trials: lessons from the SAINT I study of NXY-059 for acute ischemic stroke. Stroke. 2006;37:2644–7. doi: 10.1161/01.STR.0000241106.81293.2b. [DOI] [PubMed] [Google Scholar]
- 26.Massie CA, Hart RW, Peralez C, Richards GN. Effects of humidification on nasal symptoms and compliance in sleep apnea patients using continuous positive airway pressure. Chest. 1999;116:403–8. doi: 10.1378/chest.116.2.403. [DOI] [PubMed] [Google Scholar]
- 27.Waters KA, Everett FM, Bruderer JW, Sullivan CE. Obstructive sleep apnea: The use of nasal CPAP in 80 children. Am J Respir Crit Care Med. 1995;152:780–5. doi: 10.1164/ajrccm.152.2.7633742. [DOI] [PubMed] [Google Scholar]
- 28.Marcus CL, Ward SL, Mallory GB, et al. Use of nasal continuous positive airway pressure as treatment of childhood obstructive sleep apnea. J Pediatr. 1995;127:88–94. doi: 10.1016/s0022-3476(95)70262-8. [DOI] [PubMed] [Google Scholar]
- 29.Guilleminault C, Pelayo R, Clerk A, Leger D, Bocian RC. Home nasal continuous positive airway pressure in infants with sleep- disordered breathing. J Pediatr. 1995;127:905–12. doi: 10.1016/s0022-3476(95)70026-9. [DOI] [PubMed] [Google Scholar]