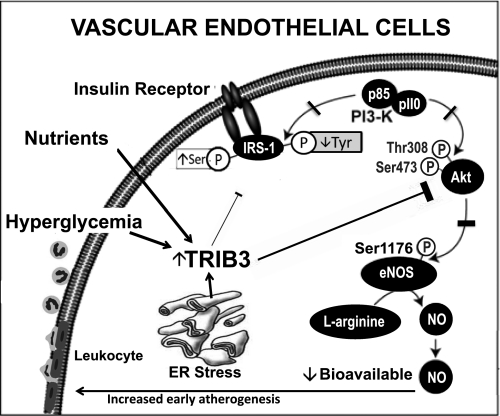
Obesity caused by excess feeding (overnutrition) has become a problem of epidemic proportions and is the underlying cause in metabolic disorders and chronic diseases such as diabetes and cardiovascular disease. Overnutrition is associated with systemic and tissue-related insulin resistance, an abnormality that promotes vascular disease as well as the development of diabetes (1,2). Thus, there is considerable interest in factors that link overnutrition, insulin resistance, and hyperglycemia with vascular disease. There is emerging evidence that the expression of the Tribbles homolog 3 of Drosophila (TRIB3) gene is increased in patients and animals with type 2 diabetes (3). The TRIB3 gene is located on the 20p13 region of the human chromosome. Its full-length translated mRNA is 1,074 base pairs, and its protein product is made up of 358 amino acids. Studies have shown that TRIB3 inhibits insulin metabolic signaling in liver (4–6), skeletal muscle (7), and vascular tissue (8). Further, these studies suggest that TRIB3 expression in skeletal muscle and liver tissue is increased with excessive nutrient intake as well as by hyperglycemia (3–7). Endoplasmic reticulum stress has also been shown to increase TRIB3 gene expression, and TRIB3 promotes cell death in response to endoplasmic reticulum stress (9) (Fig. 1). Several studies have shown that TRIB3 impairs insulin metabolic signaling by increasing serine phosphorylation of insulin receptor 1 (IRS-1), reducing tyrosine phosphorylation of this docking protein and activation of phosphatidylinositol 3-kinase and downstream protein kinase B (Akt) phosphorylation/activation (10,11) (Fig. 1). TRIB3 has also been reported to bind to and directly inhibit Akt phosphorylation/activation and to interfere with FoxO1 regulation of Akt activation (4–7). Reduced insulin-stimulated Akt activation is explained by reduced stimulation of phosphorylation at both Thr308 and Ser473 residues, which appear to be due to increased physical interaction of TRIB3 with the pleckstrin homology domain of Akt. These observations suggest that TRIB3 acts as a nutrient sensor that mediates cell stress responses under conditions of excessive nutrient intake, insulin resistance, and/or hyperglycemia (12–19).
FIG. 1.
Role of TRIB3 in impaired insulin metabolic signaling in endothelial cells. TRIB3 leads to leukocyte cell adhesion, which is an initiating event in atherogenesis. ER, endoplasmic reticulum; P, phosphorylation; PI3-K, phosphatidylinositol 3-kinase; Tyr, tyrosine.
In the current issue of Diabetes, Wang et al. (20) have evaluated the role of TRIB3 in the development of atherosclerosis and plaque stability in young (3-week-old) ApoE−/−/LDLR−/− mice that were made diabetic by a combination of high-fat and high-sugar diet and low-dose streptozotocin treatment. The strategy used to evaluate the role of TRIB3 was to silence the TRIB3 gene via intravenous adenoviral gene delivery of TRIB3 siRNA. At age 20 weeks, the diet- and streptozotocin-induced diabetic mice displayed insulin resistance, hyperglycemia, increased aortic TRIB3 gene expression, and increased macrophage migration, adhesion, and phagocytosis. The increase in gene expression of TRIB3 is consistent with prior observations that TRIB3 is upregulated in skeletal muscle from patients with type 2 diabetes, db/db mice, and Zucker fatty rats (7). Further, diabetic mice displayed more aortic, carotid, and brachiocephalic atherosclerotic plaques and increased intimal medial thickness. Knockdown of TRIB3 increased Akt phosphorylation, reduced blood glucose, increased liver glycogen content, and decreased abnormal macrophage activity as well as the number and fragility of atherosclerotic lesions (20). In this regard, TRIB3 was previously observed to be upregulated in atherosclerotic unstable plaques (19).
There are several mechanisms by which increased TRIB3 may promote atherosclerotic lesions. For example, it has been reported that a TRIB3 gain of function variant is associated with impaired insulin-mediated nitric oxide (NO) production in human endothelial cells (8). Insulin normally increases endothelial NO synthase (eNOS) activity via IRS-1/Akt signaling (2). Insulin, via Akt activation, normally stimulates Ser1177 phosphorylation, resulting in an increased flux through the reductase domain and, consequently, enhanced eNOS activity (8). In contrast, eNOS Thr495 constitutive phosphorylation downregulates eNOS activity. Overexpression of TRIB3 impairs insulin modulation of eNOS Ser1177 phosphorylation and Thr495 dephosphorylation, thus decreasing insulin’s ability to activate eNOS (8). As previously discussed, TRIB3 may inhibit this metabolic signaling pathway through increased serine phosphorylation of IRS-1 or by directly inhibiting phosphorylation/activation of Akt (2,4–7) (Fig. 1). Decreased bioavailable NO and endothelial dysfunction, which is common in insulin-resistant states, obesity, and diabetes, is an important early step in atherosclerotic development (2,8). For example, reduced bioavailable NO is associated with increased leukocyte adhesion to endothelial cells (Fig. 1), an important early step in atheroma formation (2,8).
As reviewed in the current article (20), TRIB3 is upregulated by oxidized LDL, and upregulated TRIB3, in turn, promotes increased macrophage migration, adhesion, and apoptosis, which promote the formation of unstable plaque lesions. In this regard, the diabetic animals in this study demonstrated vulnerable plaques with relatively thin fibrous caps and larger lipid cores; this abnormality was corrected with TRIB3 silencing. Thus, in diabetic states increased TRIB3 expression in the vasculature is not only an increased plaque burden but also promotes plaque instability. Given the epidemic of obesity and diabetes, and their role in promoting cardiovascular disease, molecular targeting of TRIB3 appears to have considerable potential to decrease atherosclerotic disease and acute coronary events that are seen more frequently in diabetic patients. The current work (20) suggests that the silencing of TRIB3 may provide a therapeutic strategy to lessen the burden of atherosclerotic disease in patients with the metabolic syndrome and type 2 diabetes. Additionally, hygienic measures such as reductions in caloric and alcohol intake (1–3,14) and increased exercise appear to reduce skeletal muscle TRIB3 and improve systemic and tissue insulin sensitivity. Further studies should be directed to understanding how TRIB3 interacts with other stress- and nutrient-driven molecules such as mammalian target of rapamycin, which are also negative regulators of insulin metabolic signaling (2) and vascular disease.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health grants (R01 HL73101-01A and R01 HL107910-01) and the Veterans Affairs Merit System (0018) to J.R.S.
No potential conflicts of interest relevant to this article were reported.
The author thanks Brenda Hunter, University of Missouri, for her editing assistance.
Footnotes
See accompanying original article, p. 463.
REFERENCES
- 1.Ahima RS. Digging deeper into obesity. J Clin Invest 2011;121:2076–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whaley-Connell A, Sowers JR. Indices of obesity and cardiometabolic risk. Hypertension 2011;58:991–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oberkofler H, Pfeifenberger A, Soyal S, et al. Aberrant hepatic TRIB3 gene expression in insulin-resistant obese humans. Diabetologia 2010;53:1971–1975 [DOI] [PubMed] [Google Scholar]
- 4.Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science 2003;300:1574–1577 [DOI] [PubMed] [Google Scholar]
- 5.Lima AF, Ropelle ER, Pauli JR, et al. Acute exercise reduces insulin resistance-induced TRB3 expression and amelioration of the hepatic production of glucose in the liver of diabetic mice. J Cell Physiol 2009;221:92–97 [DOI] [PubMed] [Google Scholar]
- 6.Wang YG, Shi M, Wang T, et al. Signal transduction mechanism of TRB3 in rats with non-alcoholic fatty liver disease. World J Gastroenterol 2009;15:2329–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Wu X, Franklin JL, et al. Mammalian Tribbles homolog 3 impairs insulin action in skeletal muscle: role in glucose-induced insulin resistance. Am J Physiol Endocrinol Metab 2010;298:E565–E576 [DOI] [PMC free article] [PubMed]
- 8.Andreozzi F, Formoso G, Prudente S, et al. TRIB3 R84 variant is associated with impaired insulin-mediated nitric oxide production in human endothelial cells. Arterioscler Thromb Vasc Biol 2008;28:1355–1360 [DOI] [PubMed] [Google Scholar]
- 9.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J 2005;24:1243–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004;306:457–461 [DOI] [PubMed] [Google Scholar]
- 11.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006;313:1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi L, Heredia JE, Altarejos JY, et al. TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism. Science 2006;312:1763–1766 [DOI] [PubMed] [Google Scholar]
- 13.Liew CW, Bochenski J, Kawamori D, et al. The pseudokinase tribbles homolog 3 interacts with ATF4 to negatively regulate insulin exocytosis in human and mouse beta cells. J Clin Invest 2010;120:2876–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He L, Simmen FA, Mehendale HM, Ronis MJ, Badger TM. Chronic ethanol intake impairs insulin signaling in rats by disrupting Akt association with the cell membrane: role of TRB3 in inhibition of Akt/protein kinase B activation. J Biol Chem 2006;281:11126–11134 [DOI] [PubMed]
- 15.Prudente S, Scarpelli D, Chandalia M, et al. The TRIB3 Q84R polymorphism and risk of early-onset type 2 diabetes. J Clin Endocrinol Metab 2009;94:190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prudente S, Hribal ML, Flex E, et al. The functional Q84R polymorphism of mammalian Tribbles homolog TRB3 is associated with insulin resistance and related cardiovascular risk in Caucasians from Italy. Diabetes 2005;54:2807–2811 [DOI] [PubMed] [Google Scholar]
- 17.Gong HP, Wang ZH, Jiang H, et al. TRIB3 functional Q84R polymorphism is a risk factor for metabolic syndrome and carotid atherosclerosis. Diabetes Care 2009;32:1311–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang YY, Wang ZH, Zhang LP, et al. TRB3, upregulated by ox-LDL, mediates human monocyte-derived macrophage apoptosis. FEBS J 2009;276:2752–2761 [DOI] [PubMed] [Google Scholar]
- 19.Deng J, James CH, Patel L, et al. Human tribbles homologue 2 is expressed in unstable regions of carotid plaques and regulates macrophage IL-10 in vitro. Clin Sci (Lond) 2009;116:241–248 [DOI] [PubMed]
- 20.Wang Z-h, Shang Y-y, Zhang S, et al. Silence of TRIB3 suppresses atherosclerosis and stabilizes plaques in diabetic ApoE−/−/LDL receptor−/− mice. Diabetes 2012;61:463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]