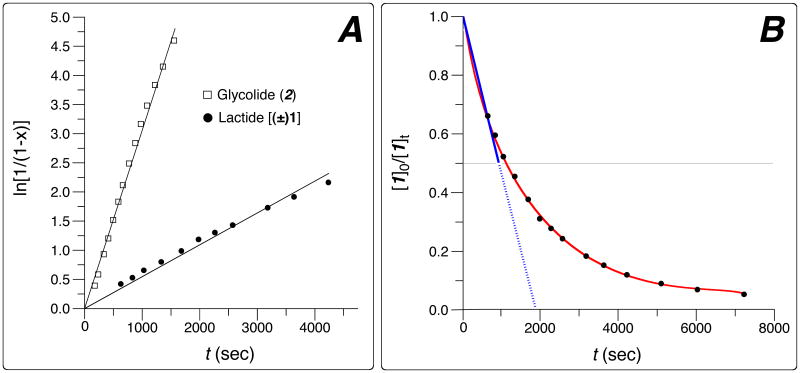
Figure 1.
Lactide and glycolide homopolymerizations in CDCl3 solvent at ambient temperature (reaction progress measured by 1H NMR spectroscopic analysis) under the following conditions (cf. Table 1). For lactide [(±)-1]: {[mPEG2k] = 5.0mM; [(±)-1]0:[mPEG2k]:[DBU] = 264:1:1.32}; for glycolide (2): {[mPEG2k] = 5.1mM, [2]0:[mPEG2k]:[DBU] = 2.94:1:0.0066. Panel A: Plot of ln[1/(1-x)] vs. time (x = monomer conversion). Panel B: Experimentally observed (red) exponential decay of monomer concentration for lactide polymerization. The blue line denotes the approximate linear conversion during the first half-life of lactide consumption, which we then used to guide the choice of the (constant) rate of glycolide addition during subsequent syntheses of the PEG-b-PLGA copolymers.