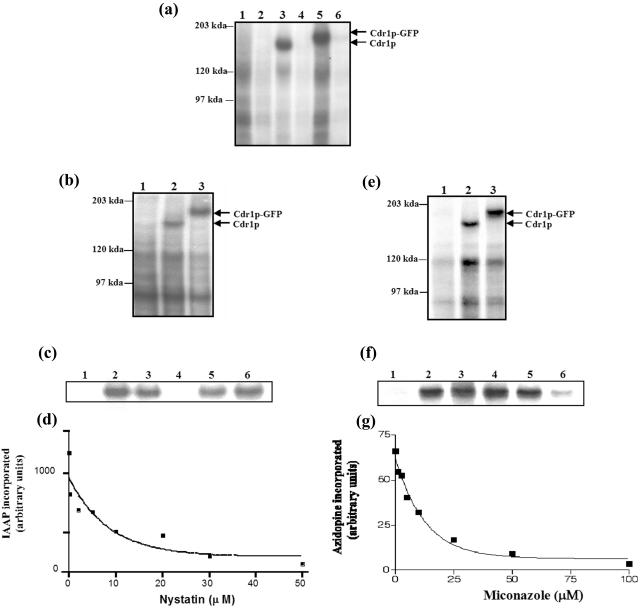
FIG. 2.
[α-32P]8-azido-ATP, [125I]IAAP, and [3H]azidopine bind specifically to Cdr1p and Cdr1p-GFP. (a) Binding of [α-32P]8-azido-ATP to Cdr1p and Cdr1p-GFP. The PM proteins (300 μg/ml) were photoaffinity labeled with 10 μM [α-32P]8-azido-ATP (10 μCi/nmol) under subdued light as described in Materials and Methods. Ice-cold ATP (10 mM) was added to displace excess bound [α-32P]8-azido-ATP where indicated. Lane 1, AD1-8u− cells; lane 2, AD1-8u− cells plus 10 mM ATP; lane 3, AD1002 cells; lane 4, AD1002 cells plus 10 mM ATP; lane 5, PSCDR1-GFP cells; lane 6, PSCDR1-GFP cells plus 10 mM ATP. (b) Binding of [125I]IAAP to Cdr1p and Cdr1p-GFP. The PM proteins (300 μg/ml) from AD1-8u− (lane 1), AD1002 (lane 2), and PSCDR1-GFP (lane 3) cells were incubated with 3 to 6 nM [125I]IAAP (2200 Ci/mmol) for 5 min under subdued light and processed as described in Materials and Methods. (c) Effects of Cdr1p substrates on the binding of IAAP to Cdr1p-GFP. The PM proteins (15 μg/50 μl) from AD1-8u− (control) (lane 1) or PSCDR1-GFP (lanes 2 to 6) cells were incubated with 100 μM concentrations of the following drug substrates for 10 min at 37°C in 50 mM Tris-HCl (pH 7.5). Lane 2, no drug; lane 3, cycloheximide; lane 4, nystatin; lane 5, anisomycin; lane 6, miconazole. The samples were brought to room temperature, and 3 to 6 nM [125I]IAAP (2200 Ci/mmol) was added and incubated for an additional 5 min under subdued light. The samples were then illuminated with a UV lamp (365-nm wavelength) for 10 min and processed as described in Materials and Methods. (d) Concentration-dependent inhibition of IAAP binding to Cdr1p-GFP by nystatin. The PM proteins (15 μg/50 μl) of PSCDR1-GFP cells were incubated with increasing concentrations (0.25 to 50 μM) of nystatin for 10 min at 37°C in 50 mM Tris-HCl (pH 7.5). The samples were brought to room temperature, and 3 to 6 nM [125I]IAAP (2200 Ci/mmol) was added and incubated for an additional 5 min under subdued light. The samples were processed and the radioactivity incorporated into the Cdr1p band was quantified as described in Materials and Methods. The data were fitted using the software GRAPHPAD PRISM 2.0 for the PowerPC Macintosh and are representative of three independent experiments. (e) Photoaffinity labeling of Cdr1p and Cdr1p-GFP with [3H]azidopine. The PM proteins (30 μg) from AD1-8u− (lane 1), AD1002 (lane 2), and PSCDR1-GFP (lane 3) cells were incubated with 0.5 μM [3H]azidopine (60 Ci/mmol) for 5 min under subdued light. The samples were processed and analyzed as described in Materials and Methods. (f) Effects of Cdr1p substrates on the binding of [3H]azidopine to Cdr1p-GFP. The PM proteins (30 μg) of AD1-8u− (control) (lane 1) or PSCDR1-GFP (lanes 2 to 6) cells were incubated with 100 μM concentrations of the following drug substrates for 10 min at 37°C in 50 mM Tris-HCl (pH 7.5). Lane 2, no drug; lane 3,cycloheximide; lane 4, nystatin; lane 5, anisomycin; lane 6, miconazole. The samples were brought to room temperature, and 0.5 μM [3H]azidopine was added and incubated for an additional 5 min, and the samples were processed as described in Materials and Methods. (g) Concentration-dependent inhibition of [3H]azidopine binding to Cdr1p-GFP by miconazole. The PM proteins (30 μg) from PSCDR1-GFP cells were incubated with increasing concentrations (0.25 to 100 μM) of miconazole for 10 min at 37°C. The samples were brought to room temperature, and 0.5 μM [3H]azidopine was added and incubated for an additional 5 min. The samples were processed and quantitated as described in Materials and Methods and the legend to Fig. 2d.