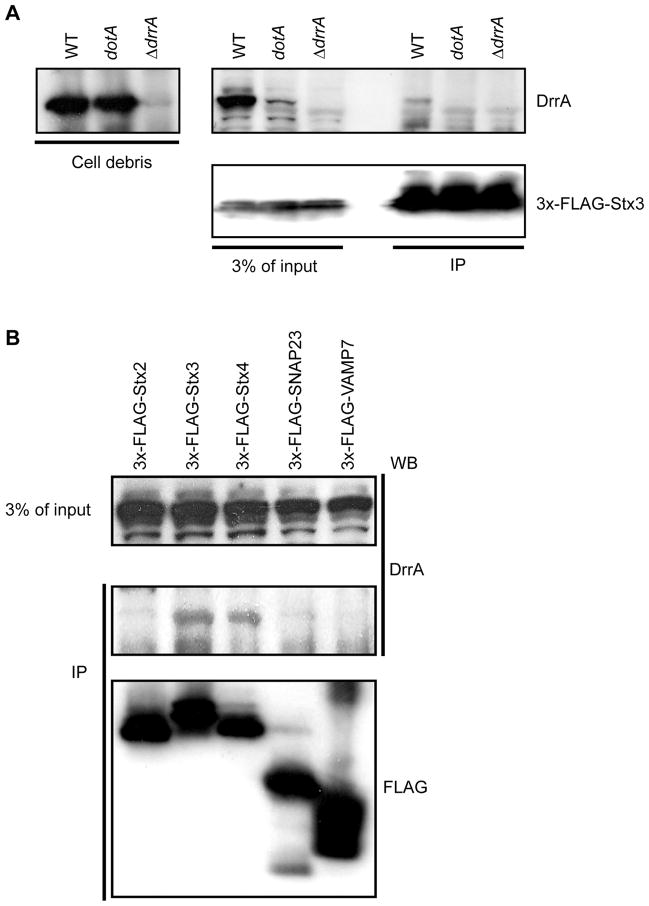
Figure 2. DrrA translocated into host cells during infection associates with PM syntaxins.
FLAG-tagged syntaxin proteins produced in HEK293-FcγRII were infected with Legionella and precipitated using anti-FLAG agarose. Immunoblot analysis was used to determine the amount of DrrA translocated into cells during infection that coprecipitated with the FLAG-tagged syntaxins. (A) HEK293-FcγRII cells producing 3x-FLAG-Stx3 were infected with wild type Legionella (WT), a Dot/Icm-deficient mutant (ΔdotA) or a mutant deficient in the DrrA protein (ΔdrrA) as indicated above each lane. Intact intracellular bacteria were collected in the pellet after centrifugation of the cell lysate and the 3x-FLAG-Stx3 was precipitated from the cleared lysate using anti-FLAG agarose. Immunoblots show the amount of DrrA protein inside the intracellular bacteria (left panel). The top right anti-DrrA blot and the bottom right anti-FLAG blot show the amount of DrrA and 3xFLAG-Stx3 in the cleared host cell lysate (3% of input) and in the 3x-FLAG-Stx3 immunoprecipitate (IP), respectively. (B) HEK293-FcγRII cells producing the indicated 3x-FLAG-tagged SNARE proteins were infected with wild type Legionella and proteins were precipitated from a lysate containing host cytosolic proteins using anti-FLAG agarose. The amount of translocated DrrA in the cleared lysate (3% of input) and the amount of the 3x-FLAG-tagged protein and DrrA protein in the immunoprecipitate (IP) was determined by immunoblot analysis.