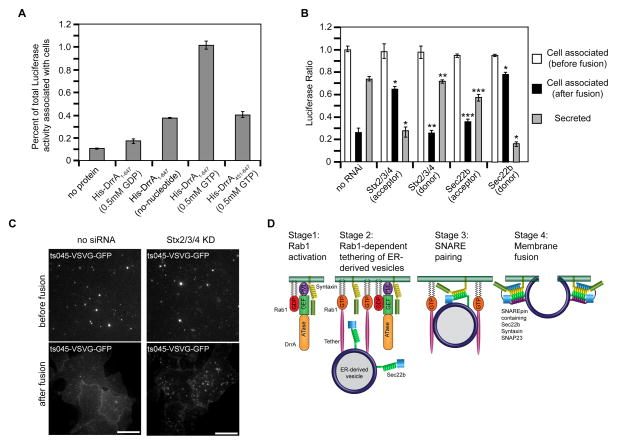
Figure 7. SNARE programming by DrrA and Rab1 promotes fusion of ER-derived vesicles with the PM.
Tethering and fusion of ER-derived vesicles containing Luciferase-KDEL or ts045-VSVG-GFP with the PM of permeabilized HEK293-FcγRII cells was measured in vitro. (A) Permeabilized HEK293-FcγRII cells were incubated with purified His-DrrA protein either in the presence or absence of nucleotides as indicated. After the addition of a PNS fraction from cells producing Luciferase-KDEL, the tethering of ER-derived vesicles was assessed by determining the Luciferase activity associated with the permeabilized cells, which is presented as the percent of total Luciferase activity added to the reaction. (B) Permeabilized HEK293-FcγRII cells were incubated with purified His-DrrA1-647 protein and a PNS fraction from cells producing Luciferase-KDEL was added. Tethering and fusion of ER-derived vesicles was assessed independently in parallel wells. The tethering of ER-derived vesicles was assessed by determining the Luciferase activity associated with the permeabilized cells, which is presented as the percent of total Luciferase activity added to the reaction (white bars, cell associated before fusion). Fusion was assessed by comparing the Luciferase activity that remained cell associated after the addition of fusion buffer (black bars) to the Luciferase activity that was liberated into the supernatant (gray bars, secreted). Shown are reactions in which siRNA was used to reduce the levels of the proteins Stx2, Stx3, and Stx4 (Stx2/3/4) or Sec22b in the permeabilized cells (acceptor), and reactions in which siRNA was used to reduce the levels of the proteins Stx2, Stx3, and Stx4 (Stx2/3/4) or Sec22b in the (donor) cells producing Luciferase-KDEL. These data are presented as a ratio of the total Luciferase activity that was associated with cells before fusion. Data are the means ± SEM of three independent experiments. *p<0.05 when compared to the same fraction in no RNAi samples; **p<0.01 when compared to the same fraction in Stx2/3/4 (acceptor) samples; ***p<0.01; compared to the same fraction in Sec22b (donor) samples. (C) Vesicles prepared from cells 15 min after ts045-VSVG-GFP was released from the ER were added to permeabilized control cells (no siRNA) or permeabilized cells in which PM syntaxins had been silenced (Stx2/3/4 KD). TIRF images show tethered ts045-VSVG-GFP-positive vesicles at the PM (before fusion) and the change in ts045-VSVG-GFP fluorescence that occurred after the addition of fusion buffer (after fusion). (D) A model that depicts data on how DrrA could promote the tethering and fusion of ER-derived vesicles with a PM-derived organelle. The first stage shows DrrA recruiting Rab1-GDP to the membrane and associating with Stx3. In the second stage the GEF activity of DrrA activates Rab1 on the PM-derived organelle, and active Rab1 is required for the association of ER-derived vesicles displaying Sec22b. A tethering factor that binds to active Rab1 is predicted to be involved. In the third stage Sec22b and Stx3 interactions result from vesicle tethering. In the final stage the fusion of ER-derived vesicles with the PM-derived organelle membrane is mediated by the assembly of a functional SNARE complex (SNAREpin) consisting of the v-SNARE Sec22b and the t-SNARE comprised of Stx3 and SNAP23.