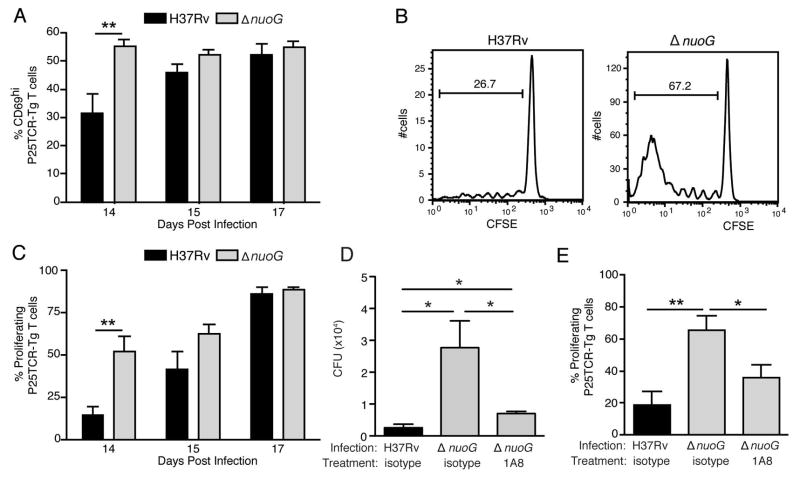
Figure 5.
The proapoptotic ΔnuoG mutant is associated with earlier activation of naïve M. tuberculosis Ag85B-specific CD4 T cells in vivo. Mice were infected one day after adoptive transfer of CFSE-labeled naïve P25TCR-Tg CD4+ T cells. (A) CD4 T cell activation as indicated by CD69 surface expression on P25TCR-Tg cells in the mediastinal lymph node on days 14, 15, and 17 post infection. (B) Representative CFSE dilution/cell proliferation profiles of adoptively-transferred P25TCR-Tg CD4+ cells in mediastinal lymph nodes of mice infected with H37Rv or ΔnuoG, 14 days post infection. The bars indicate the percentage of P25TCR-Tg cells that had undergone one or more cycles of proliferation. (C) Quantitation of of P25TCR-Tg CD4+ T cell proliferation in the mediastinal lymph nodes of groups of mice infected with H37Rv or ΔnuoG. (D) Neutrophil depletion abrogates the enhanced trafficking of M. tuberculosis in mice infected with ΔnuoG. Infected mice treated as in panels A and B received the Ly6G-specific neutrophil-depleting antibody 1A8 or isotype control antibody on day 8 post infection, and M. tuberculosis colony-forming units were quantitated on day 14 postinfection. (E) Neutrophil depletion abrogates the accelerated proliferation of Ag85B-specific CD4 T cells in mice infected with ΔnuoG. P25TCR-Tg CD4+ T cell proliferation in the mediastinal lymph nodes of the same mice as in panel D. Data in panels A–E are mean ± SD of five mice per group and time point, where A–C is representative of three separate experiments, and D and E of two separate and independent experiments. *, p < 0.05; **, p < 0.01.